研究成果 Research Results
- TOP
- News
- Research Results
- Efficient Synthesis of Unnatural alpha-Amino Acid Derivatives via Asymmetric Alkynylation
Efficient Synthesis of Unnatural alpha-Amino Acid Derivatives via Asymmetric Alkynylation
2016.05.19Research ResultsPhysics & Chemistry
α-Tetrasubstituted α-amino acids are important building blocks for peptide-based drugs from the viewpoint of their biological stability. However, the development of synthetic methods with high atom economy and broad functional group tolerance has been limited in success.
Professor Ohshima, Assistant Professor Morimoto and co-workers developed Rh-catalyzed asymmetric alkynylation that provides unnatural α-tetrasubstituted α-amino acid derivatives in high yield with high atom economy. The reaction is compatible with various functional groups. In addition, mechanistic insights disclosed would shed light on the future studies on direct catalytic reactions.
This research has been published on April 20, 2016, in the online edition of Journal of the American Chemical Society.
For more information about this research, see;
Mechanistic Studies and Expansion of the Substrate Scope of Direct Enantioselective Alkynylation of α-Ketiminoesters Catalyzed by Adaptable (Phebox)Rh(III) Complexes.
J. Am. Chem. Soc. 2016, ASAP (DOI: 10.1021/jacs.6b01590)
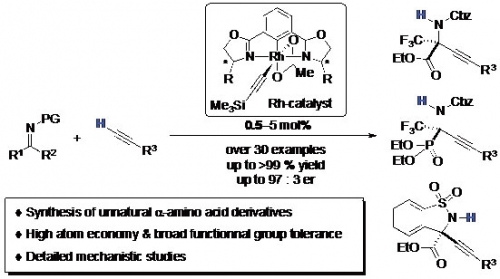
Fig. 1
Journal Reference
Mechanistic Studies and Expansion of the Substrate Scope of Direct Enantioselective Alkynylation of α-Ketiminoesters Catalyzed by Adaptable (Phebox)Rhodium(III) Complexes, ,Journal of the American Chemical Society,Research-related inquiries
- TOP
- News
- Research Results
- Efficient Synthesis of Unnatural alpha-Amino Acid Derivatives via Asymmetric Alkynylation































