
|
Seiji Shinkai Professor, Department of Chemistry and Biochemistry, Graduate School of Engineering |
- From the Nano-sized Molecular World to Huge COE Projects -
The First gMolecular Nano-Machineh Was Born in 1979
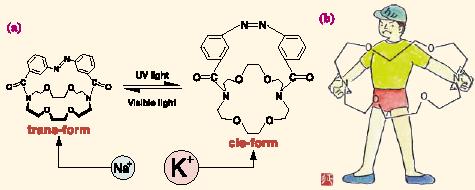
It has been a long-standing dream of scientists to manipulate nano-sized molecules in the same way we manipulate meter-sized or centimeter-sized machines. To achieve this, it was necessary to develop some kind of intelligent nano-sized gspring.h We noticed that an azobenzene group which is known to change the configuration (between trans-form and cis-form) in response to photoirradiation would be useful as a photoresponsive spring. The first example of such molecular nano-machines (published in 1979) is illustrated in Fig. 1a: therein, an oxygen-containing ring (so-called gcrown etherh) which shows the metal ion affinity is bridged by an azobenzene group. The crown ether in trans-form has a stretched, oval-shaped ring structure which shows the specific affinity with smaller metal ion Na+, whereas the cisform has a round-shaped ring structure which shows the specific affinity with the larger metal ion K+. One may conclude, therefore, that the nano-sized ring is manipulated by a nano-sized photo-expander (Fig. 1b). Thereafter, a number of molecular nano-machines exhibiting dynamic chemical and physical functions have been designed by us and others. In 2000, Balzani (University of Bologna) and Stoddart (UCLA) proposed the definition of gmolecular nano-machinesh in a review article. In that article, they recognized that our photoresponsive system published in 1979 was the first example demonstrating this concept.
|
|
Fig. 1 The first example of gmolecular nano-machinesh to manipulate molecules (a) and its cartoon image (photo-expander) (b). |
Where We Were: Rational Approach vs. Random Approach in Precise gMolecular Recognitionh
In 1981 JST (Japan Science and Technology Corporation) initiated an innovative research project called ERATO (Exploratory Research for Advanced Technology) for fostering the creation of advanced science and technology while stimulating future interdisciplinary scientific activities. The funding for ERATO projects is generous, with grants of approximately \ 1.7 billion (approximately US$ 16 million) administered for each five-year project. Although this may seem like a dream-ticket to most scientists, only 3-4 new projects are selected from all scientific research fields. SHINKAI Chemirecognics Project (1990-1995) studied the grational designh of host molecules having the precise recognition ability for various guest molecules while aiming to establish gartificialh high-precision chemirecognition systems comparable to those found in living systems. The establishment of a methodology for artificial recognition systems will make a substantial contribution to the creation of superior molecular sensors, artificial enzymes, new types of separation systems, and a variety of intelligent elements with high precision and selectivity, etc. The two representative research achievements obtained in this Project are introduced below.
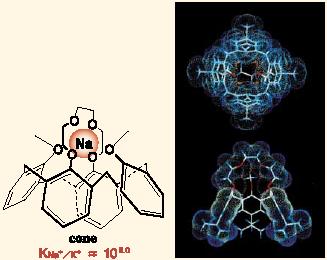
Firstly, we succeeded in developing a molecular design of calixarenes (cyclic polyphenols) that are highly selective for sodium ion. Particularly noteworthy is a calix[4]crown derivative (Fig. 2) which shows extraordinarily high sodiumion selectivity of 10^5 against the potassium record (10^3), thus providing valuable information for designing highly selective super-sensors. The calix[4]crown derivative still holds the world record for Na+ versus K+ selectivity. This selectivity is even higher than those attained by related natural antibiotics.
|
|
Fig. 2 Calixarene derivative with very high Na+ selectivity which is applicable to designing a gsuper Na+ sensorh. |
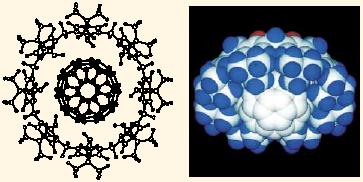
Secondly, it was found that the C60 fullerene, which has very high potential as a new functional material, can be separated from a mixture of many fullerene homologs in carbon soot utilizing a host-guest interaction with a specific calixarene. Calix[8]arene (cyclic octamer of phenols) selectively forms a complex with C60 (Fig. 3), resulting in an aggregated larger molecule that is easily precipitated and thus greatly reducing the separation costs (by two orders of magnitude). Shinkai group holds the world-wide patent, which is already used for the separation and purification of C60 in industry.
|
|
Fig. 3 Inclusion of C60 fullerene in the cavity of calix[8]arene. |
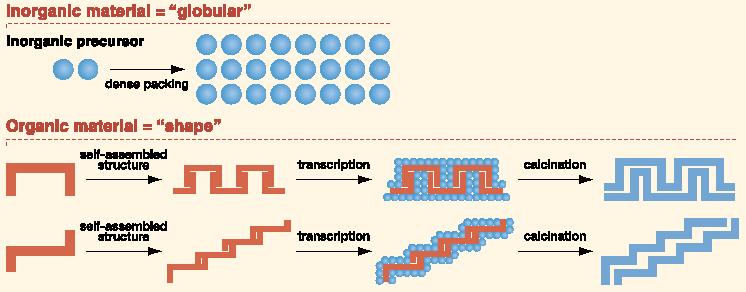
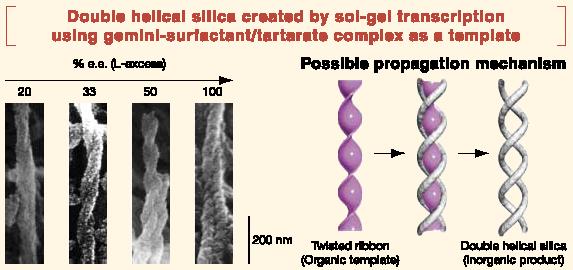
The ability to recognize metals and molecules, which living systems acquired in the process of evolution, plays an indispensable role in life processes. With the aid of this function, living systems can duly execute their inherent actions such as self-preservation, self-replication, etc. Typical examples for such actions are the interaction with receptor molecules on the cell surface, the antigen-antibody interaction, and hereditary transmission, which are all impossible to execute without highly developed recognition functions. The conventional methodology in molecular recognition chemistry had been the grational designh of the ghost hmolecules. The term grational designh refers to the process of selecting molecules which have structural characteristics complementary to the targeted gguesth metals and molecules as the staring point for designing host molecules, and then modifying them until they have the desired structure. In order to find a way to break through this impasse in molecular design, the JST-supported ICORP (International Collaboration Research Project) with Professor D. N. Reinhoudt (Fig. 4) at Twente University (The Netherlands) was launched in 1997. This project (ICORP Chemotransfiguration Project) had succeeded in exploiting the new and revolutionary concept of grandom design,h which represented a significant departure from the traditional approach of grational design.h Under the grandom designh method, molecular parts, which can interact with a guest and eventually constitute a host, are pre-organized around the guest via multi-point interaction and then immobilized into a matrix using the guest as a gtemplate.h The matrix thus assembled according to the template effect should keep an imprinted memory and be complementary to the original guest. The representative concept and results are shown in Fig. 5 and Fig. 6, respectively. Metal atoms do not have a specific gshape,h and are just densely packed when assembled. In contrast, organic molecules have a specific gshapeh and when assembled, result in a variety of different aggregate structures (called superstructures). Thus, if these gorganich superstructures are meticulously transcribed into ginorganich materials, one can create new superstructural ginorganich devices. In a sense, this research aims to compose gartificial fossilsh on a nanometer scale. It is now even possible to create a double helical superstructure, like the structure of DNA, from ginorganich compounds by transcription of an gorganich molecular assembly having a twisted ribbon structure (Fig. 6).

|
|
Fig. 4 Professor D. N. Reinhoudt and Professor S. Shinkai in front of the Imperial Palace. |
|
|
Fig. 5 Concept to make inorganic superstructures (artificial fossils) by transcription of organic superstructures. |
|
|
Fig. 6 Fibrous silica having the double helical structure created by transcription of an organic molecular assembly having a twisted ribbon structure. |
Since this novel methodology has been established through this joint research project, it is now possible to cope with the recognition of new guest molecules of any size or shape. One may view this function as constituting a kind of artificial gantibioticsh which flexibly corresponds to unknown gantigens.h After the Chemotronsfiguration Project was concluded in 2001, Professor Reinhoudt arrived at his post as a leader of MESA+, a Dutch nanotechnology project, including nearly 400 people.
Where We Are: Design and Control of Advanced Molecular Assembly Systems (MEXT COE Program)
The Ministry of Education, Culture, Sports, Science and Technology (MEXT) launched a large-scale scientific research program called the COE Project in 1995. The selection of this program was highly competitive: only 5-6 groups were selected from all scientific research fields. Once selected, however, MEXT donates ca. \ 2 billion for 5-7 years. In the division of chemistry, Nagoya University was first selected, the leader of which was Professor R. Noyori, a Nobel Prize Laureate. In the second year (1996), the chemistry group of Kyushu University was selected: as a leader of Kyushu University COE Program, Professor H. Iwamura served for the opening two years and I served for the latter five years. The project includes several big names such as Professors T. Kajiyama (presently, the President of Kyushu University), T. Kunitake, H. Okawa, Y. Naruta, M. Irie, T. Tsutsui, N. Koga, etc.
In this research project, we first established strategies for assembling and/or organizing molecules on the basis of two guiding principles that, when properly designed, molecules can attract or repel each other selectively, and that dimensionality of the structures is most instrumental in controlling the properties and functions of molecular assemblies. After the synthesis of the designed molecules the assemblage and organization of the molecules has been performed by: 1. self-assembly, 2. crystallographic technology, 3. monolayer/bilayer methods, 4. molecular manipulation by scanning probe microscopy. On the basis of these achievements, we believe that a strong research base for molecular assembly systems has been established at Kyushu University.
Where We Are Going: Functional Innovation of Molecular Informatics (MEXT 21st Century COE Program)
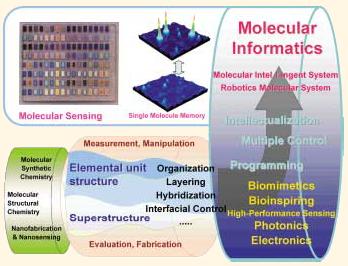
In 2002, MEXT started an innovative new program called the 21st Century COE Project. Again, the selection process for this program was highly competitive. Thanks to the aftereffect of the previous COE Project, we were selected as one of the 21 top groups in the field of chemistry and material science. The Department of Applied Chemistry and the related groups in Kyushu University have already accomplished excellent research in the field of the chemistry of sophisticated molecular materials and molecular assembly, such as synthetic bilayer membranes, guest-host molecules, photoresponsive molecules, etc. Based on such potential, this 21st Century COE program on the research and education of gFunctional Innovation of Molecular Informaticsh was organized by the member of the Department of Applied Chemistry and the related faculty members in Kyushu University. Fig. 7 summarizes the outline of this program. The ultimate aims of this 21st Century COE program are, firstly, to create a new science and technology of gmolecular informaticsh which is a key concept for the advancement of artificial molecular intelligence and molecular robotics in the 21st century and secondly, to inspire and stimulate younger generation (particularly, Ph. D. students) to participate in these innovative research activities and to let them cultivate an international way of thinking. For example, one of my former students is now working with Professor J.-M. Lehn (Nobel Prize Laureate in 1987) at University of Louis Pasteur (Fig. 8).
|
|
Fig. 7 The concept of our 21st Century COE Program, gFunctional Innovation of Molecular Informatics.h |

|
|
Fig. 8 Professor J.-M. Lehn and Dr. M. Ikeda at University of Louis Pasteur. |
According to recent statistical data, Professor S. Shinkai is ranked to the 9th in the world chemist ranking based on the number of paper citations for the past 10 years ( http://www.in-cites.com/nobel/nov2002-chemistry-top100.html ) and nominated, as one of only two Japanese chemists (along with Professor R. Noyori), to the top 100 most highly-cited chemists based on the total number of paper citations (http://isihighlycited.com//).

|
|
Mr. K. Sugiyasu (graduate student) and Professor S. Shinkai operated a scanning electron microscope to take pictures shown in Fig. 6. |