研究成果 Research Results
- TOP
- News
- Research Results
- New oral immunotherapy for Japanese cedar pollinosis using intestinal immunity
New oral immunotherapy for Japanese cedar pollinosis using intestinal immunity
2017.05.09Research ResultsLife & Health
Currently around 30% of Japanese are suffering from Japanese cedar pollinosis (JCP). This prevalence has been increasing and JCP is now considered as a part of Japanese national disease. Until now, there has been no treatment to improve immune system for those who have allergic constitutions in the short term. In this research, however, Prof.Nakayama and Assistant Prof.Daisuke Murakami of Graduate school of Medical Science, Kyushu University developed a new oral immunotherapy to improve allergic constitutions for people with JCP; that is to use intestinal immunity by taking antigen-galactomannan conjugate capsules. The treatment period is very short. For about 2 months before and after the pollen spreads by the wind, persons with JCP need to take the capsules. In the near future, there is a possibility to lead to a new immunotherapy to improve the allergy constitution.
Oral immunotherapy (OIT) using intestinal immunity with antigen-galactomannan conjugate is a novel immunotherapy that can be expected to have a therapeutic effect in a short term that could not be realized by immunotherapy currently available. In the recent RCT*1 medical experiment, OIT with antigen-galactomannan conjugate was safe and approved to reduce anti-allergic medicine by about 60% compared to standard treatment. For more information about this research, see Safety and efficacy of short-term oral immunotherapy with Cry j 1-galactomannan conjugate for Japanese cedar pollinosis: a randomized controlled trial. Sci Rep., Murakami D et al. DOI: 10.1038/srep46142.
RCT*1: Randomized Controlled Trial

Table Features of oral immunotherapy with Cry j 1-galactomannan conjugate
1. Short term regimen
2. Convenient, Minimally invasive
3. Reducing anti-allergic medicine
4. No severe systemic side effects
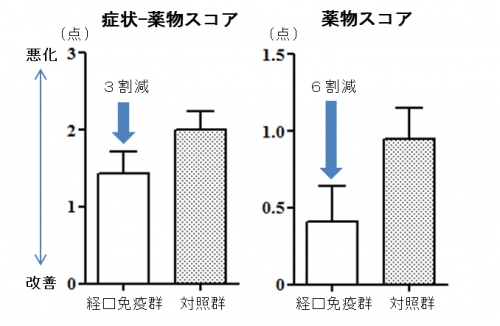
Fig. Improvements in symptom-medication score and especially medication score during pollen season were recognized with short term OIT for about 2 months.
Researcher comments
“There are some points to be improved such as internal dosage and administration period before it can be marketed as a therapeutic drug. But this breakthrough treatment may be applicable to treatment of other allergic diseases.” says Murakami D
(This research was conducted with the support of Wako Filter Technology Co., Ltd., and Japan Society for the Promotion of Science.)
Journal Reference
Safety and efficacy of short-term oral immunotherapy with Cry j 1-galactomannan conjugate for Japanese cedar pollinosis: a randomized controlled trial, ,Scientific Reports 7, 10.1038/srep46142Research-related inquiries
- TOP
- News
- Research Results
- New oral immunotherapy for Japanese cedar pollinosis using intestinal immunity































