研究成果 Research Results
- TOP
- News
- Research Results
- Developing a Catalyst for Fuel Cells That Use Hydrogen and Carbon Monoxide as Fuel
Developing a Catalyst for Fuel Cells That Use Hydrogen and Carbon Monoxide as Fuel
2017.06.23Research ResultsPhysics & ChemistryTechnologyEnvironment & Sustainability
Scientists from Kyushu University, Japan, have invented the first catalyst that can oxidize both hydrogen and carbon monoxide. Carbon monoxide is a common pollutant in commercially available hydrogen gas and it significantly reduces the activity of catalyst by poisoning the platinum catalysts used in today’s fuel cells. However, this new catalyst, based on a nickel-iridium complex, was designed to act like the mid-point between two natural enzymes: hydrogenase, which oxidizes hydrogen and carbon monoxide dehydrogenase, which oxidizes carbon monoxide. The group of scientists, led by Prof. Seiji Ogo, were then able to construct a proof-of-concept fuel cell that turned the tables on poisonous carbon monoxide and used it as a fuel and generated energy from a 50:50 mixture of the two gases.
This research achievement was published in the online edition of the German academic magazine Angewandte Chemie International Edition on June 6, 2017 (Japan local time). This research has been carried out as part of the research sponsored by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grant-in-Aid for Specially Promoted Research, “New Energy Sources from Hydrogenase-Photosynthesis Models,” by Prof. Seiji Ogo and his research group at the Center for Small Molecule Energy (Seiji Ogo, head of the center), Faculty of Engineering, Kyushu University, as well as the International Institute for Carbon-Neutral Energy Research (I2CNER, Petros Sofronis, director of the institute), base of the World Premier International Research Center Initiative (WPI) established by MEXT, JNC Corporation, and Fukuoka Industry-Academia Symphonicity.
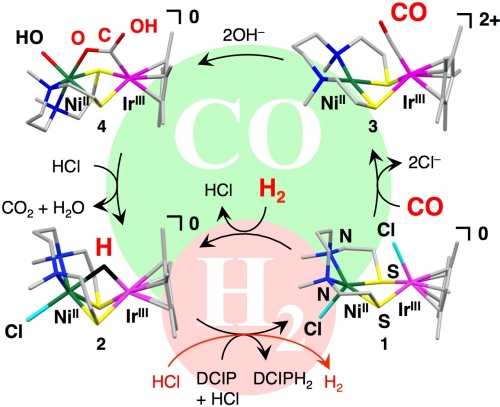
(Figure)
We developed a fuel cell that uses the anode catalyst and a 50:50 mixture of hydrogen and carbon monoxide gases as fuel.
Researcher comments
Instead of approaching the issue from the conventional standpoint of protecting the catalyst from carbon monoxide, we took a hint from two naturally occurring enzymes (carbon monoxide dehydrogenase, which oxidizes carbon monoxide, and hydrogenase, which oxidizes hydrogen) and adopted the idea to design a catalyst that would facilitate use of carbon monoxide as a fuel (electron source) in the same manner as hydrogen. Since this system works in water, it is also environmentally friendly.
Journal Reference
One Model, Two Enzymes - Activation of H2 and CO, ,Angewandte Chemie International Edition, 10.1002/anie.201704864Research-related inquiries
- TOP
- News
- Research Results
- Developing a Catalyst for Fuel Cells That Use Hydrogen and Carbon Monoxide as Fuel































