In 1984, Professor Morita participated in Physics.He was proud of his world-leading researches and pushed forward with his works constantly.
In 1984, Professor Morita participated in Physics.He was proud of his world-leading researches and pushed forward with his works constantly.
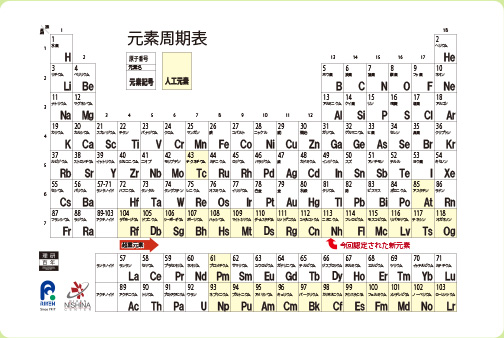 Nihonium (Nh), the official name of an atomic element 113, has been added to the Periodic Table.(As of September, 2012)
Nihonium (Nh), the official name of an atomic element 113, has been added to the Periodic Table.(As of September, 2012)
 He seeks to detect new elements using cutting-edge accelerators and separators.It is amazing that Professor Morita made those devices by his own knowledge.
He seeks to detect new elements using cutting-edge accelerators and separators.It is amazing that Professor Morita made those devices by his own knowledge.
 In 1984, Professor Morita participated in Physics.He was proud of his world-leading researches and pushed forward with his works constantly.
In 1984, Professor Morita participated in Physics.He was proud of his world-leading researches and pushed forward with his works constantly.
The main theme of my research is searching for new superheavy elements. A concept that exhibits the constituents of matter is called an element, while its substance is an atom. An atom is the smallest unit of which things are composed; all matter that exists in our world is made up of various atoms that have formed chemical bonds with each other.
 Nihonium (Nh), the official name of an atomic element 113, has been added to the Periodic Table.(As of September, 2012)
Nihonium (Nh), the official name of an atomic element 113, has been added to the Periodic Table.(As of September, 2012)
Atoms are composed of a central nucleus, surrounded by electrons. The nucleus consists of protons, which have a positive charge, and neutrons. Protons and the negatively charged electrons that surround them are attracted to each other, but protons have a mass 1,840 times greater than electrons. The atomic number of each element indicates the number of protons that it has; the higher the number, the heavier the element. The elements that occur naturally on Earth in a stable form range all the way up to uranium, whose atomic number is 92. Elements with atomic numbers higher than that must be created by synthesizing them from other elements. Success in synthesizing a new element in this way is referred to as the discovery of a new element.
 He seeks to detect new elements using cutting-edge accelerators and separators.It is amazing that Professor Morita made those devices by his own knowledge.
He seeks to detect new elements using cutting-edge accelerators and separators.It is amazing that Professor Morita made those devices by his own knowledge.
Elements with the number 104 or higher are referred to as superheavy elements; the heavier the element, the harder it is to synthesize. When protons are packed tightly into a nucleus, the electrical repulsion between the positively charged protons is very strong and the nucleus becomes unstable, making it more fragile and reducing its life. New elements are synthesized by colliding two nuclei with relatively stable lives to cause nuclear fusion. You could say that the biggest difficulty in synthesizing – that is to say, discovering – a superheavy element is the fact that a nucleus is very small, just one-trillionth of a centimeter across, and even if the two nuclei collide, the probability of their fusion is very low, only 1 in 100 trillion.
We synthesized element 113 by accelerating a beam of zinc nuclei (atomic number 30) in an accelerator and colliding it into a bismuth (atomic number 83) target. As a result of around 4 trillion collisions over nine years, we succeeded in synthesizing it three times, leading to its recognition as a new element. Elements up to atomic number 118 have now been discovered, so our team is currently engaged in ongoing experiments aimed at discovering 119 and 120. Countries across the globe compete with each other to discover new superheavy elements, so we have to be on top of our game 24 hours a day, 365 days a year. The existence of elements up to 172 has been posited in theory, but they say that in reality, 130 or so is about the limit. New accelerators and separators also need to be developed at the same time, so constant progress is required in all areas. We also know that past 172, a zone called the "island of stability" appears, which has not yet been reached by humanity. It is said that if these have a special number of protons, the element's life will increase sharply to anywhere between 300 and 500 years. The life of element 113 is two-thousandths of a second. While the path ahead of us will be long, we will of course explore its possibilities.


Hardly any discoveries in basic research have a direct impact on daily life. Digging down further in basic research does not yield knowledge that will be of use immediately, or even in the near future, but could lead to something at some distant stage in the future. The new element that I discovered through my research might benefit humanity decades or centuries from now. For example, the machines now used as a matter of course in hospitals for MRI and CT scans would not exist if our ancestors had not discovered new elements. The process of basic research itself leads to the development of science and technology and social advances.
Cutting-edge accelerators and separators are required for the discovery of new elements. Wanting to discover a new element that nobody on Earth had ever seen before, using apparatus that I had made with my own two hands, I studied mechanical engineering from scratch and designed a big piece of equipment. The heart-pounding excitement that I felt when I discovered a world-first phenomenon inside that apparatus was overwhelming. The new element that I discovered after so much toil and effort will be inscribed in the periodic table, which one could describe as a common global legend handed down since yesteryear, and passed on through future generations in perpetuity. The foundations of science were laid by humans, the only intelligent life on earth, and the periodic table is one of humankind's assets. My sense of joy and pride that one seat at that table has been occupied thanks to the work of a Japanese national is incalculable.
![]()
![]()