研究成果 Research Results
- TOP
- News
- Research Results
- Unraveling how local anesthetics numb pain
Unraveling how local anesthetics numb pain
2019.07.19Research ResultsLife & Health
Local anesthetics are vital to making medical procedures more bearable for patients around the world every day, but the detailed mechanism of how these crucial chemicals disrupt our sensation of pain is still unclear despite their extensive use. Now, researchers from Kyushu University’s Faculty of Science have evidence that points to the loosening of rigid groupings of lipid molecules in nerve membranes as a key process.
Pain signals are transmitted to our brain by nerve cells, each of which is surrounded by a membrane of lipid molecules mixed with additional molecules that impart other functionality. Channels that transport ions across the membrane are particularly important, as they create the imbalance of charges inside and outside the cell that produces electrical signals for cell communication. Thus, local anesthetic molecules have been thought to block nerve pulses by interacting with ion channels, but how this interaction occurs has been unclear.
To unravel this process, Masanao Kinoshita, assistant professor, and Nobuaki Matsumori, professor, focused on the interaction of anesthetics with rigid groupings of lipid molecules in the membrane—called lipid rafts—that move through the otherwise fluid-like membrane (Fig. 1, left) since sodium ion channels are known to be selectively recruited in lipid rafts.
Investigating the impact of three representative local anesthetics—dibucaine, tetracaine, and lidocaine—on artificial membranes with raft-like portions, the researchers found that dibucaine and tetracaine effectively disrupted the raft-like membrane domains, returning them to a state similar to the surrounding fluid-like membrane, while lidocaine hardly affected the domains.
Interestingly, the propensity of the anesthetics for disrupting the raft-like membranes was consistent with their anesthetic potency (Fig. 1, right). Their data also suggest that, although the likeliness of the more-potent dibucaine and tetracaine to incorporate themselves into the raft-like domains was unexpectedly smaller than that of lidocaine, the binding locations of dibucaine and tetracaine were deeper than those of lidocaine. Thus, how deeply the molecules penetrate into the lipid rafts appears to be more important than the number of molecules that do.
This newly proposed mechanism for how local anesthesia works will aid in forming a deeper understanding of anesthesia toward the development of new anesthetics.
For more information about this research, see “Mechanism of local anesthetic-induced disruption of raft-like ordered membrane domains,” Masanao Kinoshita, Takeshi Chitose, and Nobuaki Matsumori, Biochimica et Biophysica Acta - General Subjects (2019), https://doi.org/10.1016/j.bbagen.2019.06.008
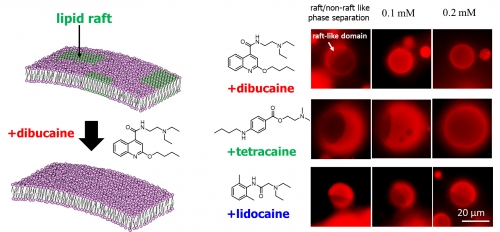
Fig. 1. (Left) A schematic illustration of lipid raft disruption by dibucaine. Green regions show lipid rafts in an otherwise disordered cell membrane. Some ion channels are recruited in lipid rafts, so disruption of the rafts can affect the ion flow and transmission of signals. (Right) Influence of local anesthetics on the separation of raft-like and non-raft-like phases of the membranes. The brighter regions show the raft-like ordered membrane domains.
Journal Reference
Mechanism of local anesthetic-induced disruption of raft-like ordered membrane domains, ,Biochimica et Biophysica Acta - General Subjects , https://doi.org/10.1016/j.bbagen.2019.06.008Research-related inquiries
- TOP
- News
- Research Results
- Unraveling how local anesthetics numb pain































