研究成果 Research Results
- TOP
- News
- Research Results
- Artificial cells offer route to explore cell processes
Artificial cells offer route to explore cell processes
2019.07.29Research ResultsLife & HealthPhysics & ChemistryTechnology
Nearly all living systems in nature share a common building block: the cell. Thus, to understand how cells work is to understand the fundamental processes for life.
Researchers from Kyushu University’s Faculty of Physics, in close collaboration with the University of Minnesota, now report a novel platform for developing artificial cells that can interact with their surroundings to better study these fundamental processes.
The research group developed a new way to enclose protein synthesis reactions in cell-sized compartments—or microwells—of 15 pL sealed by a biological phosphilipid membrane. By including a membrane similar to that which forms the boundary of real cells, the reactions inside the microwells can interact with a controlled medium on the other side of the membrane.
“Membrane-bound artificial cells are an important tool to expand our knowledge of the mechanisms that shape the living world at the smallest scales,” says Ziane Izri, first author on the ACS Synthetic Biology paper detailing the new results.
The research group, led by Yusuke T. Maeda, associate professor in the Faculty of Physics, filled the microwells with cell components extracted from E. coli cells and additional nutrients before sealing them with membranes. Inside the microwells, the E. coli extract continued the process of creating protein molecules based on the instructions in the DNA present in the microwells.
By adding DNA for a protein that glows green when illuminated with ultraviolet light, the researchers could measure the sensitivity of the protein synthesis in the membrane-bound microwells to the composition of the outer medium based on how brightly the microwells glowed.
Outer media with different compositions led to great variations in brightness after several hours, proving that the artificial cells have the ability to communicate with the outer medium.
“More advanced metabolic functions should also be possible in these bioreactors, so we are excited to explore the functionalization of the membrane through the synthesis of specific membrane proteins in the future,” comments Izri.
For more information about this research, see “Gene expression in on-chip membrane-bound artificial cells,” Ziane Izri, David Garenne, Vincent Noireaux, and Yusuke T. Maeda, ACS Synthetic Biology (2019), https://doi.org/10.1021/acssynbio.9b00247
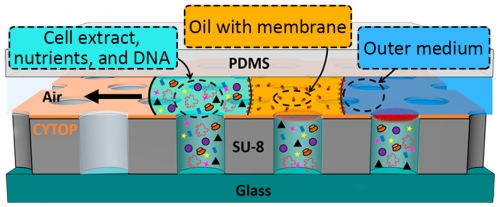
Fig. 1. Artificial cells were formed in microwell structures created on a piece of glass and separated from the outside by a membrane. The microwells were formed by consecutively flowing a cell mixture (aqua), an oil (orange) with membrane building blocks, and the outer medium (blue) over the wells to fill the well, create the membrane (red), and control the outside environment.
Journal Reference
Gene Expression in on-Chip Membrane-Bound Artificial Cells, , ACS Synthetic Biology, https://pubs.acs.org/doi/10.1021/acssynbio.9b00247Research-related inquiries
- TOP
- News
- Research Results
- Artificial cells offer route to explore cell processes































