研究成果 Research Results
- TOP
- News
- Research Results
- Greener chemistry through direct synthesis of key nitrogen-containing compounds
Greener chemistry through direct synthesis of key nitrogen-containing compounds
New catalytic synthetic method for ketimines with unprotected nitrogen atoms could simplify synthesis of important bioactive compounds and reduce waste 2020.02.19Research ResultsLife & HealthPhysics & ChemistryEnvironment & Sustainability
Researchers at Kyushu University’s Graduate School of Pharmaceutical Sciences developed a new method that could simplify the synthesis of certain nitrogen-containing organic molecules, potentially reducing the environmental impact of creating materials of interest for pharmaceuticals and medicinal chemistry.
Organic compounds containing nitrogen can be found throughout all forms of life and are central to the development and study of pharmaceuticals and other bioactive compounds. However, creating these molecules in the laboratory from basic building blocks is often a multistep process that produces various wastes and requires purification between steps.
Thus, the development of efficient synthesis methods is desirable both for increasing the amount of a compound that can be easily made and for reducing the environmental impact of the synthesis.
One common bottleneck is the need to attach a chemical group not used in the final molecule to the nitrogen atom to protect it and prevent it from participating unwanted reactions during synthesis—only to have to remove the protecting group later.
Takashi Ohshima, Hiroyuki Morimoto, and coworkers now report in Organic Letters that they have developed a new series of reactions that can be used to obtain a variety of nitrogen-containing compounds without protecting the nitrogen atom during the reactions, thereby reducing the steps.
In particular, the researchers focused on reactions that replace an oxygen atom in common organic starting materials with an unprotected nitrogen atom and allow for the next step of the synthesis to proceed without a need to purify the intermediate material.
The key intermediate materials in this case have the unprotected nitrogen atom double bonded to a carbon atom, which is in turn attached to two other chemical groups. This basic structure is called ketimine.
“We previously developed methods for simpler synthesis of nitrogen compounds from ketimines with unprotected nitrogen groups, but we soon found that its applicability was limited by the available synthetic methods for the intermediate ketimines themselves,” saysOhshima.
“Thus, we set out to develop a new method for directly synthesizing the ketimines with an unprotected nitrogen from various carbonyl compounds and found we could do it using a commercially available metal catalyst and nitrogen source.”
The new method is readily applicable for synthesizing more than ten grams of material at a time—an important step toward fabricating materials for use on a larger scale—with a minimal formation of waste.
Further, useful nitrogen-containing compounds, such as the starting materials for unnatural amino acid synthesis, could be produced through one-pot synthesis—a process in which reaction steps are performed consecutively without the need to stop and purify the intermediate materials—due to the low reactivity of the unwanted byproducts created during the unprotected ketimine synthesis.
“This is just one way that we are hoping to move toward green chemistry for pharmaceuticals that not only saves lives but is also environmentally friendly,” comments Ohshima.
For more information about this research, see “Scandium(III) triflate-catalyzed direct synthesis of N-unprotected ketimines,” Yuta Kondo, Tetsuya Kadota, Yoshinobu Hirazawa, Kazuhiro Morisaki, Hiroyuki Morimoto, and Takashi Ohshima, Organic Letters (2020), https://doi.org/10.1021/acs.orglett.9b04038
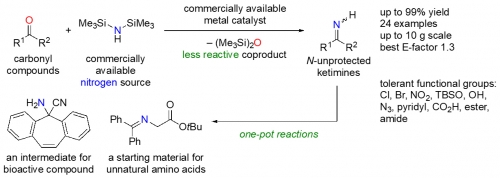
Synthesis scheme for converting carbonyl compounds into ketimines with unprotected nitrogen atoms that can be used in one-pot reactions for making nitrogen-containing organic compounds.
Journal Reference
Scandium(III) Triflate Catalyzed Direct Synthesis of N-Unprotected Ketimines, ,Organic Letters, https://doi.org/10.1021/acs.orglett.9b04038Research-related inquiries
- TOP
- News
- Research Results
- Greener chemistry through direct synthesis of key nitrogen-containing compounds































