研究成果 Research Results
- TOP
- News
- Research Results
- Life arrives at same solution using different molecular approaches
Life arrives at same solution using different molecular approaches
Study shows that two catalysts with the same function essentially employ the same technique despite being based on different molecular species 2020.12.24Research ResultsLife & Health
In a study that could provide a clue to the origin of life, an international team of researchers in Japan and the USA have elucidated interactions between proteins and transfer RNA and found that two completely different molecular species came to develop similar mechanisms.
While life has produced numerous enzymes with a huge variety of functions through evolution, examples also exist throughout biology of different catalytic molecules that can perform identical biological reactions.
One such case is a type of catalyst that plays a key role modifying the transfer RNA (tRNA) molecules that help to build proteins. Known as ribonuclease P (RNase P), the catalyst comes in two flavors: one composed of RNA molecules, referred to as ribozyme-type, and one composed only of proteins, referred to as protein-only RNase P (PRORP).
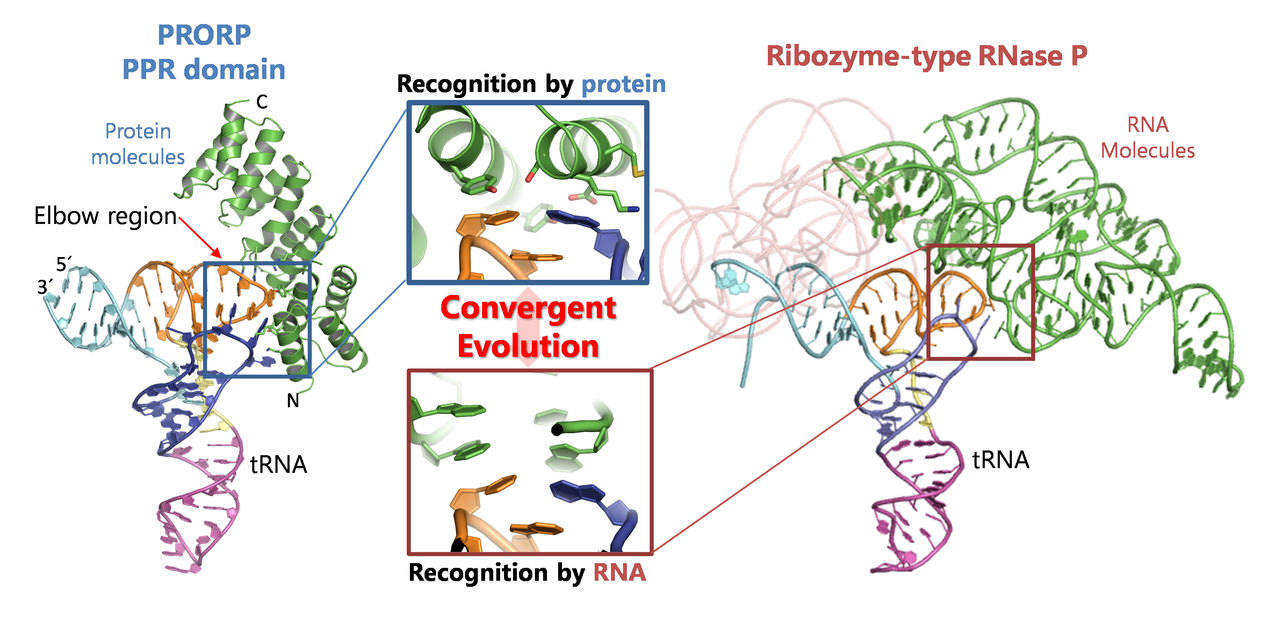
Comparison between the structure of PRORP PPR domain-tRNA complex (left), which was determined in this study, and the previously reported ribozyme-type RNase P structure (right).
This makes RNase P an excellent target for comparing catalytic strategies of independently evolved protein and RNA catalysts to understand the diversity and molecular evolution of enzymes.
Takamasa Teramoto, assistant professor, and Yoshimitsu Kakuta, professor, of Kyushu University’s Faculty of Agriculture in collaboration with the National Institute of Environmental Health Sciences (USA) and the University of Michigan (USA) now report in a study selected as a breakthrough article in Nucleic Acids Research new details on how the protein-only version interacts with tRNA.
Closely analyzing the structure of a region of PRORP—referred to as the PPR domain—that recognizes and locks on to part of the tRNA, the researchers found that the PPR domain recognizes an elbow region specific to tRNA that is already known to also be recognized by ribozyme-type RNase P.
The results indicate that, despite the two types of RNase P being based on different molecular species of RNA and protein, both recognize the same substrate in a similar way to assist the same reaction.
This phenomenon of life finding different approaches to arrive at similar solutions is an example of convergent evolution.
These findings could contribute to understanding of the origin of life, which has been hypothesized to start with RNA molecules.
###
For more information about this research, see “Pentatricopeptide repeats of protein-only RNase P use a distinct mode to recognize conserved bases and structural elements of pre-tRNA,” Takamasa Teramoto, Kipchumba J. Kaitany, Yoshimitsu Kakuta, Makoto Kimura, Carol A. Fierke, and Traci M. Tanaka Hall, Nucleic Acids Research (2020). https://doi.org/10.1093/nar/gkaa627
This release is also available in Japanese.
Research-related inquiries
Takamasa Teramoto, Assistant Professor
Faculty of Agriculture
Contact information can also be found in the full release.
- TOP
- News
- Research Results
- Life arrives at same solution using different molecular approaches































