研究成果 Research Results
- TOP
- News
- Research Results
- New cell source promising for treatment of liver diseases
New cell source promising for treatment of liver diseases
Researchers directly reprogram cells into human hepatic progenitor cells capable of proliferating and differentiating into two key cells of the liver 2021.01.22Research ResultsLife & Health
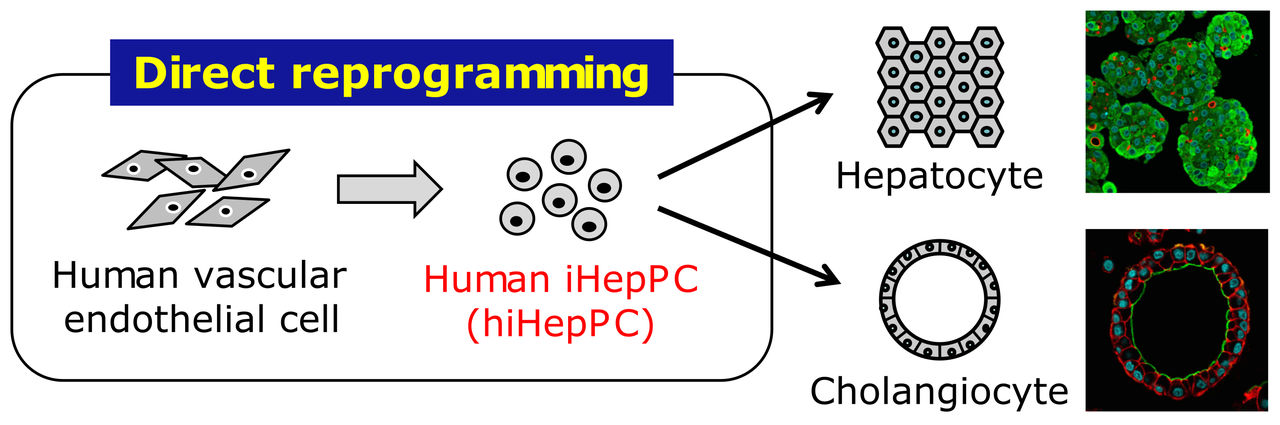
Reprogrammed from human vascular endothelial cells, hiHepPCs can stably propagate in monolayer culture and give rise to functional hepatocytes and cholangiocytes under three-dimensional culture conditions for possible application to cell transplantation therapy and drug discovery research for liver diseases.
A new method developed by researchers at Kyushu University to directly reprogram cells into progenitor cells capable of producing two key cells in the liver could one day provide a new source of cells for transplantation therapies for patients with severe liver diseases.
Indicating the strong potential for this method, liver cells created in this way produced new, functioning tissue and greatly improved survival rate when transplanted in mice with injured livers.
While the fate of the cells that make up an organism’s various organs and tissues is determined, or “programmed,” during development, researchers have made remarkable progress developing methods to reprogram cells by artificially manipulating their gene expression and the surrounding environment.
Called “direct reprogramming,” the procedure has drawn attention from around the world as a new technology for future innovative medical treatments.
Focusing on creating liver cells known as hepatocytes, the research team led by Atsushi Suzuki, professor at Kyushu University’s Medical Institute of Bioregulation, previously succeeded in reprogramming mouse fibroblasts into induced hepatocyte-like cells (iHepCs) with functional properties of liver cells by introducing two genes encoding transcription factors (Hnf4α and either Foxa1, Foxa2, or Foxa3).
Despite a similar technique being able to produce human iHepCs, the human iHepCs could not proliferate, preventing their use in applications requiring a large number of cells such as cell transplantation therapy or drug discovery research.
To solve this problem, Suzuki and his team aimed to create cells one step before hepatocytes: hepatic progenitor cells that can differentiate into both hepatocytes and bile duct epithelial cells and have higher proliferative activity.
Now, the team reports they succeeded in generating induced hepatic progenitor cells (iHepPCs) able to propagate in long-term culture by introducing a set of three transcription factors (FOXA3, HNF1A, and HNF6) into vascular endothelial cells derived from human umbilical vein- and peripheral blood-derived endothelial cells.
These iHepPCs were found to form hepatic and bile duct tissue-like structures under three-dimensional culture conditions and to have the ability to differentiate and mature into functional hepatocytes and bile duct epithelial cells.
In addition, when hepatocytes generated from human iHepPCs were transplanted into the injured livers of recipient mice models with a low survival rate of 20%, the researchers found that functioning human liver parenchyma was regenerated in the mouse liver, significantly improving the chance of survival to 80%.
The method developed in the study should allow for the procurement of large quantities of functionally mature hepatocytes and bile duct epithelial cells from human iHepPCs.
In the future, the researchers expect that the use of human iHepPC-derived hepatocytes and bile duct epithelial cells in place of liver-derived hepatocytes and bile duct epithelial cells will help realize new transplantation therapies for patients with severe liver diseases and inform the creation of a system capable of evaluating the effects and toxicity of drugs on an individual basis.
###
For more information about this research, see “Direct reprogramming of human umbilical vein- and peripheral blood-derived endothelial cells into hepatic progenitor cells,” Hiroki Inada, Miyako Udono, Kanae Matsuda-Ito, Kenichi Horisawa, Yasuyuki Ohkawa, Shizuka Miura, Takeshi Goya, Junpei Yamamoto, Masao Nagasaki, Kazuko Ueno, Daisuke Saitou, Mikita Suyama, Yoshihiko Maehara, Wataru Kumamaru, Yoshihiro Ogawa, Sayaka Sekiya, and Atsushi Suzuki, Nature Communications (2020). https://doi.org/10.1038/s41467-020-19041-z
This release is also available in Japanese.
Research-related inquiries
Atsushi Suzuki, Professor
Division of Organogenesis and Regeneration, Medical Institute of Bioregulation
Contact information can also be found in the full release.
- TOP
- News
- Research Results
- New cell source promising for treatment of liver diseases































