研究成果 Research Results
- TOP
- News
- Research Results
- Molecular traffic key to keeping cellular highways maintained
Molecular traffic key to keeping cellular highways maintained
Biomolecular motors transporting cargo within the cells found to wear down intracellular highways, prompting autonomous repair 2021.03.09Research ResultsLife & HealthMaterials
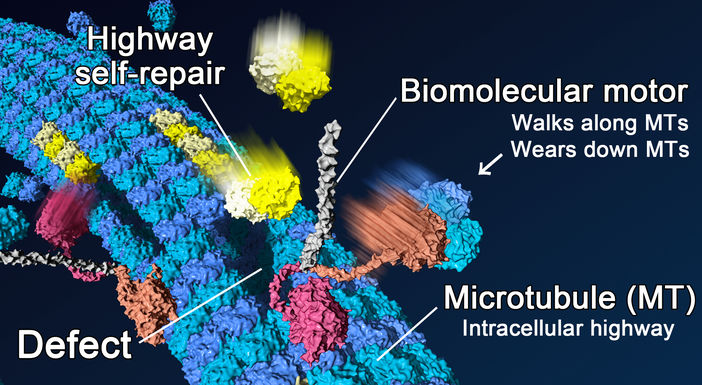
New research shows that biomolecular transport wears down microtubules (blue), which act as intracellular highways. Free tubulins (yellow) around microtubules can compensate for the damage induced by the motors (self-repair).
In living cells, biomolecular motors act as vehicles to transport intracellular cargo along molecular highways of microtubules. But while damage to typical roads caused by cars and trucks is periodically repaired by paving work, relatively little is known about the degradation and maintenance of their molecular counterparts.
Now, researchers in Japan and France studying mixtures of the biomaterials in the lab have discovered that biomolecular motors also wear down microtubule highways and that the damage is fixed through a self-repair process.
The building blocks of microtubules are tubulin heterodimers—units consisting of two different proteins. Within the microtubule shaft, tubulin units adopt a densely packed and highly ordered crystal-like lattice structure, which is generally considered to be stable.
To walk along these microtubules, biomolecular motors such as kinesin and dynein convert the chemical energy of the molecule adenosine triphosphate (ATP) into motion. However, it has been unclear how the force exerted by these biomolecular motors impacts the shaft stability and dynamics of microtubules.
In a study published in Nature Materials, researchers revealed in reconstituted systems using purified biomaterials that the mechanical action of biomolecular motors can remove tubulin from the microtubule shaft and rapidly destroy them. Moreover, this effect was not observed when free tubulins were present in the system thanks to self-repair through the insertion of new free tubulins into the areas damaged by the biomolecular motors.
In addition to preventing the destruction of microtubules traversed by biomolecular motors through the maintenance of roads that have been used, the self-repair contributes to the rejuvenation of the microtubules by replacing old tubulins in the lattice with fresh ones.
“Thanks to this mechanism, the more microtubules are traversed by biomolecular motors, the more they undergo self-repair, thus rejuvenating and strengthening them,” says Daisuke Inoue, one of the lead authors on the study and assistant professor at Kyushu University’s Faculty of Design. “Essentially, this mechanism promotes transportation and can transform frequently used small roads into highways.”
Such self-repair and rejuvenation processes are associated with many biological functions, playing roles in our health and the pathways of various diseases, so the researchers plan to explore more applications of the microtubule’s self-repair in not only biological but also medical science.
###
For more information about this research, see “Self-repair protects microtubules from their destruction by molecular motors,” Sarah Triclin, Daisuke Inoue, Jérémie Gaillard, Zaw Min Htet, Morgan E. DeSantis, Didier Portran, Emmanuel Derivery, Charlotte Aumeier, Laura Schaedel, Karin John, Christophe Leterrier, Samara L. Reck-Peterson, Laurent Blanchoin, and Manuel Théry, Nature Materials (2021). https://doi.org/10.1038/s41563-020-00905-0
This release is also available in Japanese from Kyushu U and in French at the CNRS website.
Research-related inquiries
Daisuke Inoue, Assistant Professor
Faculty of Design
Contact information can also be found in the full release.
- TOP
- News
- Research Results
- Molecular traffic key to keeping cellular highways maintained































