研究成果 Research Results
- TOP
- News
- Research Results
- Exercise reverses cognitive deficiencies caused by anesthesia
Exercise reverses cognitive deficiencies caused by anesthesia
Cognitive deficiencies caused by pediatric anesthesia in mice are due to changes in chromatin accessibility and can be reversed by exercise 2022.04.28Research ResultsLife & HealthPhysics & Chemistry
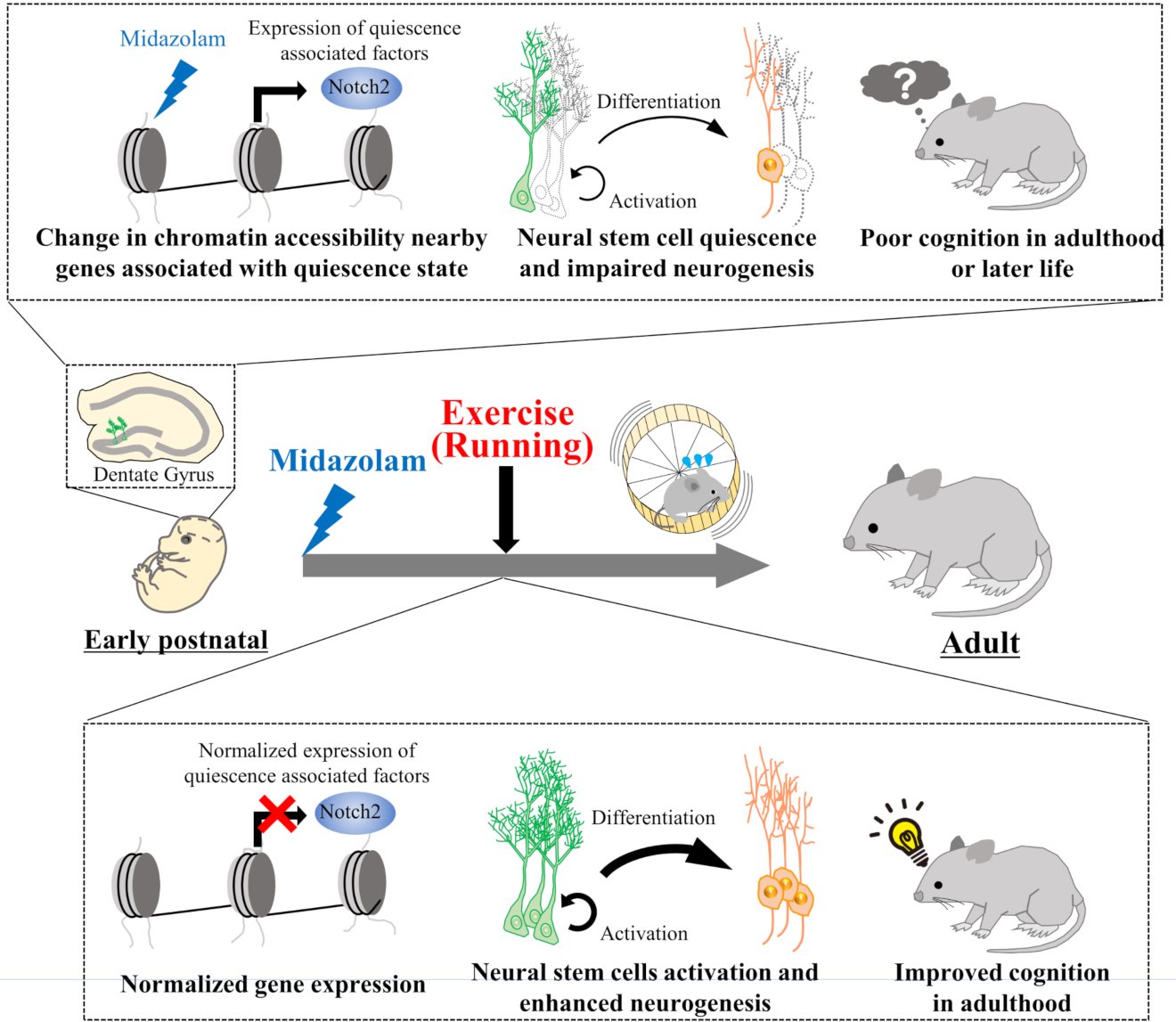
For many children, the last thing they remember in the operating room is the anesthesiologist looking down on them. Although a medically accepted practice, past research has shown that children who undergo anesthesia multiple times are at risk of developing cognitive problems—such as learning disabilities and attention deficits—later in life. However, little is known about the mechanisms and molecular changes in the brain that could be causing this.
A recent study in the Proceedings of the National Academy of Sciences of the United States of America by Kyushu University scientists gives some new clues. The team reports that alterations in the DNA and protein assembly that controls gene expression, known as chromatin, of neural stem cells was the key. The activation of the gene Egr1 following repeated exposure to an anesthetic at a young age are responsible for memory deficits in adult mice. The study further shows that voluntary running ameliorates these negative effects.
Stem cells are critical for the normal growth of all organs and for injury recovery. They proliferate, meaning they replicate into more stem cells, and differentiate, producing the various cells of our body. They also alternate between dormant and activated states depending on the requirements of the organ.
"The hippocampus is one of the major brain regions responsible for long-term memory. Neural stem cells in the hippocampal dentate gyrus are a major source of its neurons," explains Kyushu University Professor Kinichi Nakashima, who led the study.
To investigate how pediatric anesthesia affects neural stem cells during brain development, Nakashima and his colleagues administered midazolam, a common anesthetic, to one-week-old mice—the equivalent of a one-year-old child—on three consecutive days.
They found that the mice’s neural stem cells showed a higher preference for the dormant state, an effect that was still observed two months later, when the mice reached the equivalent of adulthood in humans. The dormant neural stem cells were also accompanied by abnormally low neurogenesis in the hippocampus.
"Furthermore, we found that these mice had lower performance on several memory tests. Additional investigation showed that the neural stem cells underwent epigenetic changes that increased the accessibility of genes involved in their dormancy," said Taito Matsuda, another author of the study.
Among these genes was Erg1, a transcription factor first discovered in mice and recognized for its role in memory formation and epigenetic reprogramming. Experiments by the research group showed that Erg1 reduced the proliferation and differentiation of neural stem cells and that midazolam induced the gene's expression.
"Because Erg1 is a transcription factor, its expression leads to the activation of several genes related to neural stem cell dormancy," Matsuda continued. "The subsequent chromatin remodeling allowed these genes to be persistently active even though Erg1 gene was itself activated transiently."
However, the effects could be reversed by a simple exercise program. If the mice voluntarily ran on a running wheel beginning three weeks after the midazolam exposure, the memory deficits recorded two months later disappeared.
"We suspect the running had some effect on the chromatin remodeling, and we are working on understanding that mechanism," said Nakashima.
The findings give new molecular insights into how pediatric anesthesia can lead to behavioral problems later in life and suggest a physically active lifestyle can prevent these negative effects.
###
For more information about this research see "Early-life midazolam exposure persistently changes chromatin accessibility to impair adult hippocampal neurogenesis and cognition," Hiroyoshi Doi, Taito Matsuda, Atsuhiko Sakai, Shuzo Matsubara, Sumio Hoka, Ken Yamaura, and Kinichi Nakashima, Proceedings of the National Academy of Sciences of the United States of America (2021). https://doi.org/10.1073/pnas.2107596118
This release is also available in Japanese.
Research-related inquiries
Kinichi Nakashima, Professor
Faculty of Medical Sciences
Contact information can also be found in the full release.
- TOP
- News
- Research Results
- Exercise reverses cognitive deficiencies caused by anesthesia































