研究成果 Research Results
- TOP
- News
- Research Results
- Time determines the type of cells that emerge in the mouse brain
Time determines the type of cells that emerge in the mouse brain
Scientists show that a single protein, BMP2, and epigenetics determine the type of brain cells produced from neural stem cells 2022.05.16Research ResultsLife & HealthPhysics & Chemistry
There are nearly 100 million cells in the brain, the development of which begins with the generation of neurons, the cells responsible for our thoughts and actions. Later, a different kind of cells called astrocytes form, and they modulate the performance of the neurons. But just how the brain knows when to produce these cells sequentially remains unclear.
In a new study in Genes & Development, Kyushu University researchers show that a single molecule, BMP2, triggers differentiation of neural stem cells into either neurons or astrocytes depending on the epigenetic status of the cell.
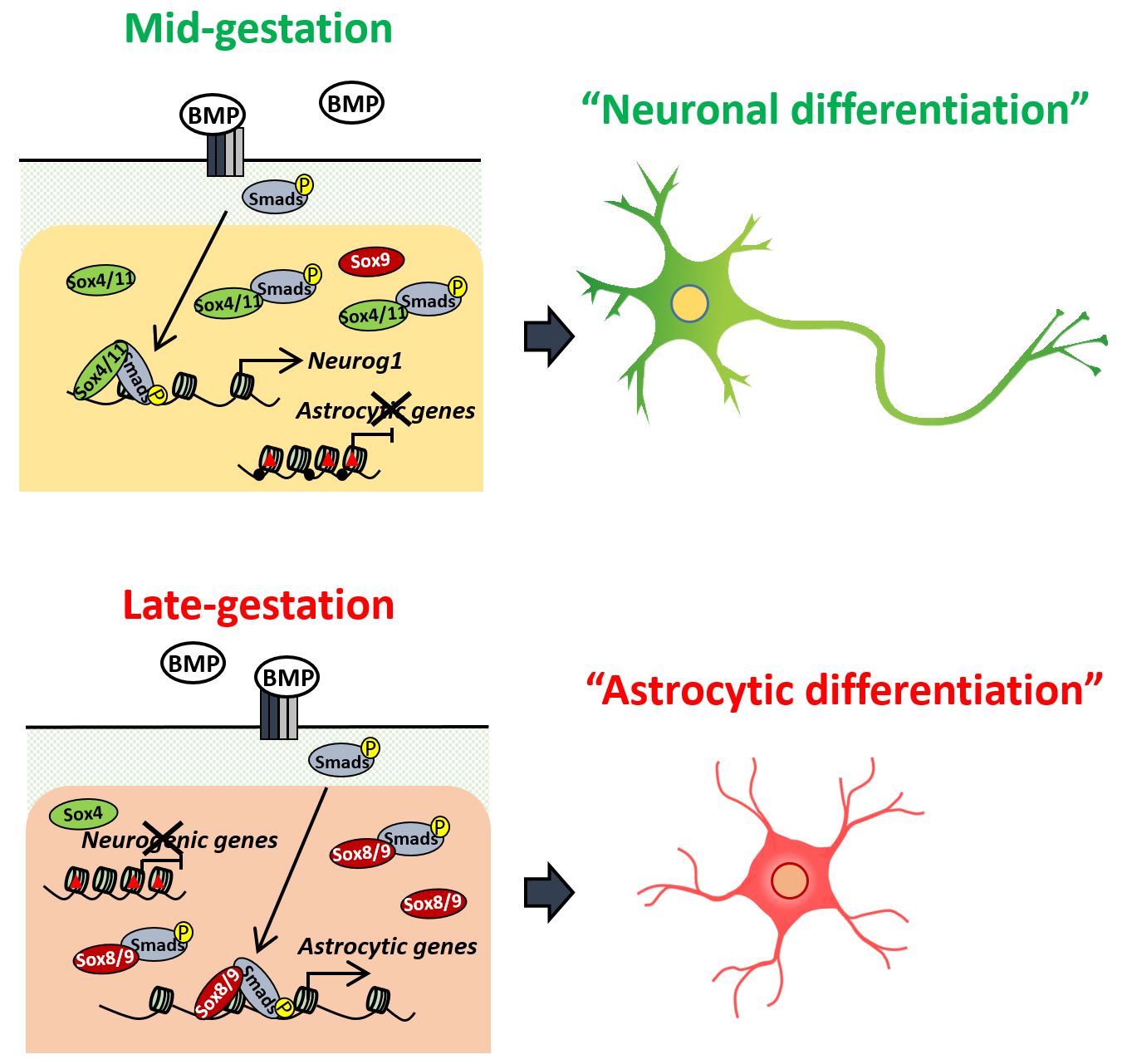
"Neural stem cells undergo repeated division and then differentiate into neurons and later into astrocytes, but how that timing is determined is not clear," explains Kyushu University Professor Kinichi Nakashima, who led the study. "One thing we do know is that the gene BMP2, or bone morphogenetic protein 2, is one of several BMPs known for promoting differentiation of neural stem cells."
BMPs induce the differentiation of neurons in mid-gestation, but they also stimulate the differentiation into astrocytes in late gestation.
To investigate the molecular cause of this switch, the research team examined neural stem cells in mouse embryos at different points in development. As expected, neural stem cells taken from mid-gestation embryos and given BMP2 would differentiate into neurons, but when taken just three days later they would differentiate into astrocytes.
"We found Smads, a common transcription factor that determines the activation or inhibition of genes, bound to different gene loci depending on the gestation period," said Sayako Katada, one of the authors of the study. "The loci were representative of genes for neuron differentiation or astrocyte differentiation."
"H3K27me3, a type of histone modification, represses gene expression. This type of modification was prevalent in Smad-binding sites for genes related to astrocytes at mid-gestation and then for genes related to neurons at late-gestation," explains Katada.
However, she noted, the pattern for DNA methylation—another type of modification that controls how genes are expressed—was not as clear. Instead, DNA methylation decreased for astrocyte genes with time but did not change so much for genes related to neurons.
Nakashima says the realization that changes occur in how cells respond to BMP2 over time provides a mechanistic explanation for how different brain cells are formed in the developing brain.
"Our study shows the interplay between cell-extrinsic and cell-intrinsic factors on neural stem cells that generate the appropriate number of brain cells during development," he concludes.
###
For more information about this research, see "Neural stem/precursor cells dynamically change their epigenetic landscape to differentially respond to BMP signaling for fate switching during brain development," Sayako Katada, Jun Takouda, Takumi Nakagawa, Mizuki Honda, Katsuhide Igarashi, Takuya Imamura, Yasuyuki Ohkawa, Shoko Sato, Hitoshi Kurumizaka, and Kinichi Nakashima, Genes & Development (2021). https://doi.org/10.1101/gad.348797.121
This release is also available in Japanese.
Research-related inquiries
Kinichi Nakashima, Professor
Faculty of Medical Sciences
Contact information can also be found in the full release.
- TOP
- News
- Research Results
- Time determines the type of cells that emerge in the mouse brain