研究成果 Research Results
- TOP
- News
- Research Results
- Highly active and chemoselective iron catalyst for transesterification was developed
Highly active and chemoselective iron catalyst for transesterification was developed
2016.06.23Research ResultsPhysics & Chemistry
We developed a highly active and chemoselective dinuclear iron(III) catalyst for transesterification. Using this iron catalyst, O-selective transesterification of “aromatic” amino alcohols and transesterification of various tertiary alcohols such as tert-butanol were achieved for the first time.
This research achievement has been published on June 16, 2016, in the online edition of the German academic magazine Chemistry –A European Journal.
Prof. Ohshima says that the present iron catalyst is highly useful for the synthesis of artificial peptides and functional polymers.
For more information about this research, see
Chemistry –A European Journal, Ohshima et al.: “μ-Oxo-Dinuclear Iron(III) Catalyzed O-Selective Acylation of Aliphatic and Aromatic Amino Alcohols and Transesterification of tert-Alcohols”
DOI: 10.1002/chem.201602801
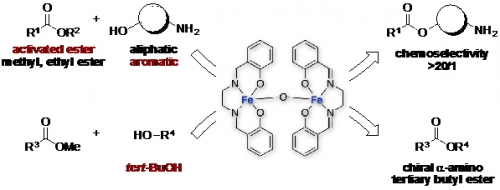
Fig. 1. μ-Oxo-Dinuclear Iron(III) Catalyzed O-Selective Acylation
We developed a chemoselective O-acylation with activated esters in the presence of amines catalyzed by μ-oxo-dinuclear iron(III) salen complex. The present catalysis was applicable not only to functionalized amino alcohols, but also to less reactive aminophenols, which is unprecedented. Furthermore, our iron complex catalyzed the transesterification of various tertiary alcohols, including tert-BuOH, for the first time
Journal Reference
μ-Oxo-Dinuclear Iron(III) Catalyzed O-Selective Acylation of Aliphatic and Aromatic Amino Alcohols and Transesterification of tert-Alcohols, ,Chemistry –A European Journal, 10.1002/chem.201602801Research-related inquiries
- TOP
- News
- Research Results
- Highly active and chemoselective iron catalyst for transesterification was developed































