研究成果 Research Results
- TOP
- News
- Research Results
- Successful development of new helical molecules showing excellent catalytic performance
Successful development of new helical molecules showing excellent catalytic performance
2016.11.08Research ResultsPhysics & Chemistry
The rational design and development of new chiral ligands to enable stereocontrol in a wide variety of reactions is one of the most important topics in organic synthesis. Over the past few decades, a large number of privileged chiral ligands with central, axial or planar chirality were developed.
Assistant Professor Usui, Dr. Yamamoto, Professor Hiral, Professor Emeritus Suemune and co-workers developed a series of novel optically active helicene-derived phosphine ligands. These ligands were highly effective in the palladium catalyzed asymmetric reactions (allylic substitutions and Suzuki-Miyaura coupling reaction).This result may open a new avenue toward the application of helicene-based ligands in metal-catalyzed asymmetric reactions. This study was collaborated with Professor Tomooka and Assistant Professor Igawa of the Institute for Materials Chemistry and Engineering at Kyushu University.
The outcomes of this study were published on November 08, 2016, in the online journal of Scientific Reports.
For more information about this research, see; Rational Design and Synthesis of [5]Helicene-Derived Phosphine Ligands and Their Application in Pd-Catalyzed Asymmetric Reactions. Sci. Rep. 2016 (DOI:10.1038/srep36211)
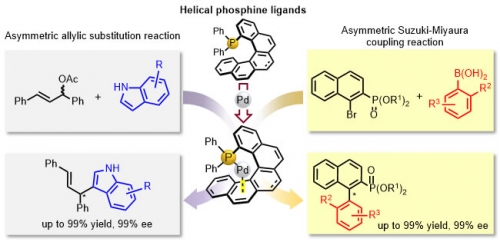
Pd-catalyzed asymmetric allylic substitution reaction and Suzuki-Miyaura coupling reaction using helical phosphine ligand.
Journal Reference
Rational Design and Synthesis of [5]Helicene-Derived Phosphine Ligands and Their Application in Pd-Catalyzed Asymmetric Reactions, ,Scientific Reports, 10.1038/srep36211Research-related inquiries
- TOP
- News
- Research Results
- Successful development of new helical molecules showing excellent catalytic performance































