研究成果 Research Results
- TOP
- News
- Research Results
- Transglutaminase is secreted via an unconventional mechanism involving exosomes and two types of fatty acylations
Transglutaminase is secreted via an unconventional mechanism involving exosomes and two types of fatty acylations
2017.05.17Research ResultsLife & Health
Transglutaminases (TGs) play pivotal roles in covalent crosslinking between specific proteins both inside and outside of the cells such as to formulate skin/cuticle, to coagulate blood/hemolymph, and to regulate the signaling pathways. The TGs identified so far do not have a typical N-terminal or internal signal sequence for secretion, and the molecular mechanisms for TGs subcellular localization and secretion are not understood. In this study, the research groups of Assistant Professor Toshio Shibata and Professor Shun-ichiro Kawabata investigated the fruit fly Drosophila and found that two types of fatty-acid: N-myristoylation and S-palmitoylation modify TG-A. TG-A is one of the two alternative splicing forms of TGs and it control not only its localization on the plasma membrane through an unconventional secretory pathway, but also its secretion in response to external stimuli such as bacterial infection. Moreover, ultracentrifugation and electron microscopic analyses, and treatments with inhibitors for multivesicular body (MVB) formation revealed that TG-A is secreted by exosomes, the extracellular vesicles. The 8-residue N-terminal fragment of TG-A containing the fatty acylation sites was both necessary and sufficient for the exosome-dependent secretion of TG-A. In conclusion, TG-A is secreted through an unconventional ER/Golgi-independent pathway involving two types of fatty acylations and exosomes.
For more information about this research, see Drosophila TG-A transglutaminase is secreted via an unconventional Golgi-independent mechanism involving exosomes and two types of fatty acylations. (DOI: 10.1074/jbc.M117.779710)
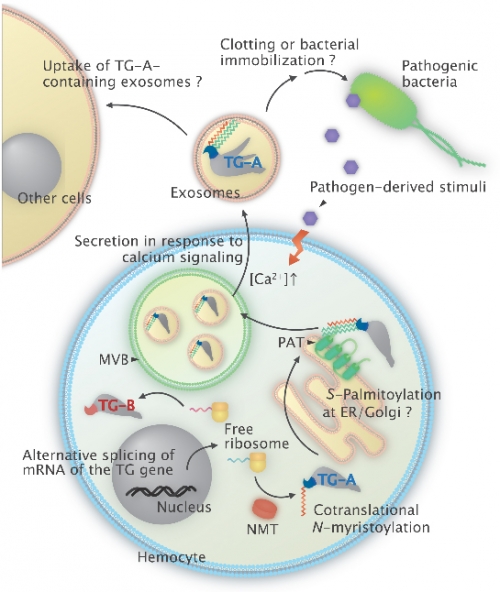
Figure: A schematic model of an estimated intracellular transport and secretion pathway of TG-A through an unconventional secretory route, including the two types of fatty-acid modifications.
At first, TG-A is co-translationally N-myristoylated in the cytosol and subsequently sorted to the cytoplasmic surface of the ER/Golgi, where S-palmitoylation occurs by membrane-bound palmitoyl acyl-transferases. Following the transient association with the cytoplasmic surface of the ER/Golgi, TG-A is modified by both N-myristoylation and S-palmitoylation, then is recruited into the MVBs. Finally, TG-A is stored by exosome in response to external stimuli, such as pathogenic bacteria.
Researcher comments
Journal Reference
Drosophila TG-A transglutaminase is secreted via an unconventional Golgi-independent mechanism involving exosomes and two types of fatty acylations, ,The Journal of Biological Chemistry, 10.1074/jbc.M117.779710Research-related inquiries
- TOP
- News
- Research Results
- Transglutaminase is secreted via an unconventional mechanism involving exosomes and two types of fatty acylations































