研究成果 Research Results
- TOP
- News
- Research Results
- World’s first demonstration of persistent luminescence from organic materials set to unlock new and expanded uses for the glow-in-the-dark phenomenon
World’s first demonstration of persistent luminescence from organic materials set to unlock new and expanded uses for the glow-in-the-dark phenomenon
2017.10.12Research ResultsPhysics & ChemistryMaterialsTechnology
Glow-in-the-dark paints that have improved flexibility and transparency while also being cheaper and easier to manufacture are on the horizon courtesy of new research from Kyushu University. In a groundbreaking demonstration, light emission lasting more than one hour was achieved from organic materials, which may also unlock new applications such as in bio-imaging because of their unique properties.
Based on a process called persistent luminescence, glow-in-the-dark materials work by slowly releasing energy absorbed from ambient light. Used in watches and emergency signs, most commercial glow-in-the-dark materials are based on inorganic compounds and include rare metals such as europium and dysprosium. However, these materials are expensive, require high temperatures to manufacture, and scatter light—as opposed to being transparent—when ground into powders for paints.
Carbon-based organic materials—similar to those used in plastics and pigments—can overcome many of these disadvantages. They can be excellent emitters and are already widely used in organic light-emitting diodes (OLEDs)¹. But achieving long-lived emission has been difficult, and the longest emission from organics under indoor lighting at room temperature was only a few minutes.
Researchers at Kyushu University’s Center for Organic Photonics and Electronics Research have now overcome this limitation by using simple mixtures of two appropriate molecules. By melting together molecules that donate electrons and ones that accept electrons—referred to as donor and acceptor molecules—emission lasting for over an hour was demonstrated for the first time without the need for intense light sources or low temperatures.
Absorption of light by an acceptor molecule gives the molecule extra energy that it can use to remove an electron from a donor molecule. The extra electron on the acceptor molecule can then hop to other acceptor molecules and move away from the positively charged donor molecule, resulting in separation of the charges. The separated charges gradually come back together—some slowly and some more quickly—and release their energy as light ¬over the span of almost an hour, resulting in the glow-in-the-dark effect.
The mixtures and processes are similar to what are found in organic solar cells² and OLEDs. After building up separated charges like in a solar cell, the charges have nowhere to escape, so they eventually comeback together to emit light like an OLED. The key difference in the newly developed mixtures is that the charges can exist in a separated state for very long periods of times.
Challenges still remain, but a new wave of glow-in-the-dark materials based on organics look poised to invigorate the area and expand their applications. The organic materials can be easily synthesized and processed, so large scale use will become cheaper. Organics present routes to transparent panels and flexible films or fibers, and the formulation of paints also appears simpler. The researchers are now looking into new molecule structures to increase the emission duration and efficiency as well as to change the color.
For more information about this research, see “Organic long persistent luminescence,”Ryota Kabe and Chihaya Adachi, Nature. DOI: 10.1038/nature24010

(left) Chihaya Adachi, Professor and Ryota Kabe, Assistant Professor
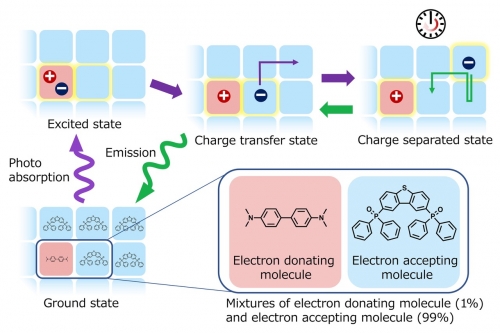
Mechanism of persistent luminescence from organic materials
[Glossary]
(1) Organic light-emitting diode (OLED): A device that employs thin films of organic materials to convert electricity into light. Charges injected from the device’s electrodes recombine in the organic thin films to produce light.
(2) Organic solar cell: A device that employs thin films of organic materials to convert light into electricity. Most organic solar cells use a combination of donor and acceptor molecules to achieve the energy conversion.
Researcher comments
Just by mixing two kinds of organic molecules, we found that persistent luminescence was possible. This organic system is extremely promising because properties such as color and emission duration can potentially be varied over a wide range simply by making small changes in the choice of molecules.
Journal Reference
Organic long persistent luminescence, ,Nature, 10.1038/nature24010Research-related inquiries
- TOP
- News
- Research Results
- World’s first demonstration of persistent luminescence from organic materials set to unlock new and expanded uses for the glow-in-the-dark phenomenon