研究成果 Research Results
- TOP
- News
- Research Results
- Development of a benzylic thiolation reaction under Weakly Acidic Conditions: A New option for Bioconjugation Reaction
Development of a benzylic thiolation reaction under Weakly Acidic Conditions: A New option for Bioconjugation Reaction
2018.03.01Research ResultsPhysics & Chemistry
Conjugation between natural functional groups in biomolecules and synthetic compounds is called bioconjugation reaction. Many scientists are interested in these reactions since it has various applications such as the synthesis of biological tools for elucidating biological functions of living organisms, preparing antibody-drug conjugates, and constructing libraries of bioactive molecules. In many cases, bioconjugation reactions with the thiol-reactive compound have been established and applied to the functionalization of bioactive peptides and proteins, however, the development of suitable reactions for bioconjunction remains difficult since there were some concerns regarding oxidation of thiol to disulfide, and competition with amine nucleophile at high pH.
In this study, Professor Takeshi Oshima and Research Fellow Kenji Watanabe utilized the activation of benzylic hydroxyl group and succeeded to find the new approach to the reaction based on the hypothesis that benzylic substitutions of appropriately designed alcohols could be used for a selective bioconjugation with thiol at a weakly acidic pH, where oxidation of thiols did not occur. A benzylic substitution of 3-indolyl(hydroxyl)acetate derivatives with thiols proceeded specifically in the presence of amino, carboxy, and phosphate groups in weakly acidic aqueous solutions under nearly physiological condition, while no reaction occurred at pH over 7. The thioether linkage formed by the reaction was stable, and did not cause exchange of thiols by treatment with excess external thiols. Moreover, the application of the present method for functionalization of biomacromolecules was demonstrated using various proteins. This is expected to advance disclosure of information, drug discovery, and the development of reaction that is biocompatible.
The above results have been published online on February 19, 2018 in Chemistry—A European Journal, an international general chemistry journal published by the Wiley, and selected as a Hot Paper and a Frontispiece of the journal.
For more information about this research, see: Bioconjugation with Thiols by Benzylic Substitution K. Watanabe, T. Ohshima, Chem.—Eur. J. Early View (DOI: 10.1002/chem.201706149).
This study was supported by JSPS KAKENHI(JP 15H05846) and (JP16K17902)
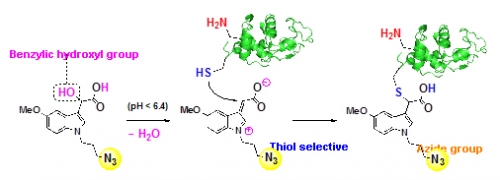
◇ Features ◇
✔ The reaction gives water as the only co-product, it is highly biocompatible.
✔ ︎ Oxidation of thiol and side reaction with amine do not occur
✔ Various functional molecules can be introduced through the azide group
Professor Takashi Ohshima and Research Fellow Kenji Watanabe
Journal Reference
Bioconjugation with Thiols by Benzylic Substitution, ,Chemistry - A European Journal, 10.1002/chem.201706149Research-related inquiries
- TOP
- News
- Research Results
- Development of a benzylic thiolation reaction under Weakly Acidic Conditions: A New option for Bioconjugation Reaction































