研究成果 Research Results
- TOP
- News
- Research Results
- Serine protease zymogen Prochelicerase C is intermolecularly activated through a lipopolysaccharide-induced active transition state
Serine protease zymogen Prochelicerase C is intermolecularly activated through a lipopolysaccharide-induced active transition state
2018.06.22Research ResultsLife & HealthPhysics & Chemistry
The research group led by Prof. Shun-ichiro Kawabata and Assoc. Prof. Toshio Shibata at Kyushu University’s Faculty of Science succeeded to confirm the presence of an active transition state of Prochelicerase C induced by interactions with LPS.
Horseshoe crab hemolymph coagulation is initiated by the autocatalytic activation of a serine protease zymogen Prochelicerase C to the active form, α-Chelicerase C. The autocatalytic activation of Prochelicerase C has been believed to be triggered through an active transition state of Prochelicerase C responding to bacterial lipopolysaccharide (LPS), designated Prochelicerase C*. However, the existence of Prochelicerase C* is only speculative and its proteolytic activity has not been validated. Moreover, it remains unclear whether the proteolytic cleavage of the Phe-737–Ile-738 bond (F737 site) of Prochelicerase C required for the conversion to α-Chelicerase C occurs intramolecularly or intermolecularly.
Here the group shows that the F737 site of a catalytic Ser-941-deficient mutant of Prochelicerase C is LPS-dependently hydrolyzed by an F737 site-uncleavable mutant, clearly indicating the existence of the active transition state of Prochelicerase C without cleavage of the F737 site. Moreover, found that the autocatalytic cleavage of Prochelicerase C occurs intermolecularly between Prochelicerase C* molecules on the LPS surface.
For more information about this research, see Intermolecular autocatalytic activation of serine protease zymogen factor C through an active transition state responding to lipopolysaccharide.
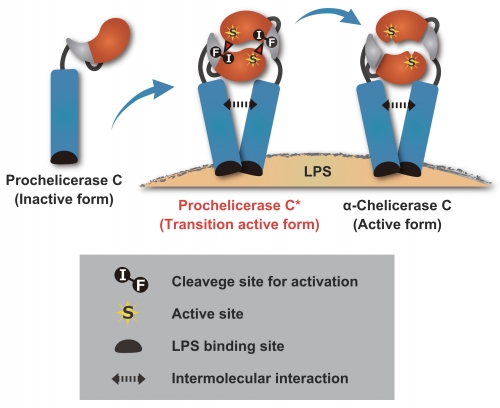
Figure:
A proposed model for the autocatalytic activation of Prochelicerase C on LPS. Coagulation factor Prochelicerase C, a precursor form, is secreted from hemocytes at injured sites in response to the LPS stimulation derived from Gram-negative bacteria. The autocatalytic activation and the initiation of the coagulation cascade occurs through the active transition state Prochelicerase C* on the lipopolysaccharide surface.
Journal Reference
Intermolecular autocatalytic activation of serine protease zymogen factor C through an active transition state responding to lipopolysaccharide, ,The Journal of Biological Chemistry, 10.1074/jbc.RA118.002311Research-related inquiries
- TOP
- News
- Research Results
- Serine protease zymogen Prochelicerase C is intermolecularly activated through a lipopolysaccharide-induced active transition state































