研究成果 Research Results
- TOP
- News
- Research Results
- New mechanism of pain control revealed
New mechanism of pain control revealed
Discovery of a distinct group of astrocytes in the spinal cord overturns thinking on role of descending neurons in pain transmission 2020.11.25Research ResultsLife & Health
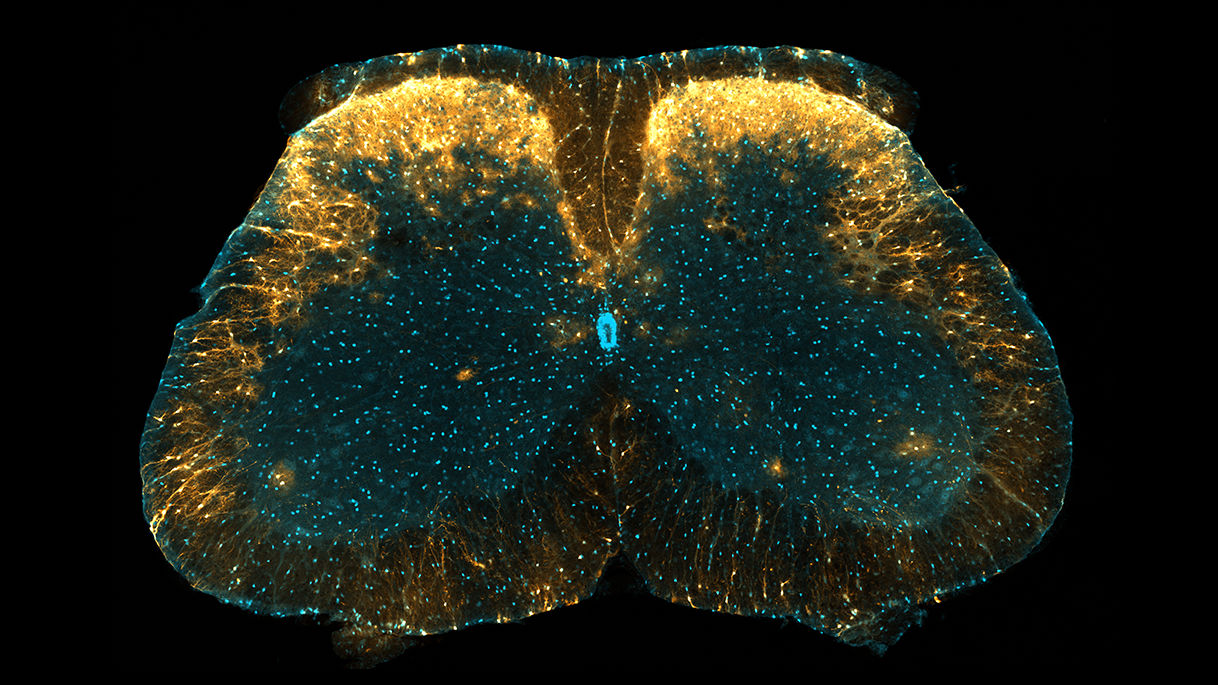
Marked as concentrated yellow here, a unique population of astrocytes in the dorsal horn of the mouse spinal cord have been found to play a role in controlling pain.
Researchers in Japan have revealed a previously unknown mechanism for pain control involving a newly identified group of cells in the spinal cord, offering a potential target for enhancing the therapeutic effect of drugs for chronic pain.
While neurons may be the most well-known cells of the central nervous system, an assortment of non-neuronal cells first discovered in the mid-nineteenth century also play a wide variety of important roles.
Originally named after the Greek word for “glue,” these glial cells are now known to be much more than glue and in fact are critical elements for regulating neuronal development and function in the central nervous system.
Among the different types of glial cells, astrocytes are the most abundant in the central nervous system, but, unlike neurons in different brain regions, researchers still have yet to develop a detailed understanding of groupings of astrocytes with distinct properties.
Now, researchers led by Makoto Tsuda, professor at Kyushu University’s Faculty of Pharmaceutical Sciences, have discovered a unique population of spinal cord astrocytes with a role in producing pain hypersensitivity.
Found in the outer two layers of gray matter near the back of the spinal cord—a location referred to as the superficial laminae of the spinal dorsal horn—the astrocytes are in a region known to carry general sensory information such as pressure, pain, and heat from around the body to the brain.
Using mice, the researchers showed that stimulating noradrenergic (NAergic) neurons—so called for their use of noradrenaline as a neurotransmitter—that carry signals from the locus coeruleus (LC) in the brain down to the spinal dorsal horn activates the astrocytes and that the astrocyte activation results in pain hypersensitivity.
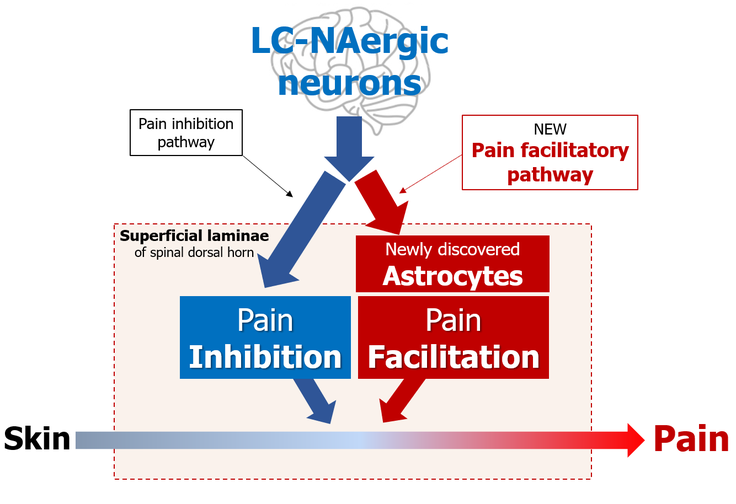
Descending LC-NAergic neurons are well known to suppress pain transmission in the spinal dorsal horn. However, researchers uncovered a new role of descending LC-NAergic neurons in facilitating spinal pain transmission by identifying a new population of spinal cord astrocytes.
These observations overturn the prevailing view that descending LC-NAergic neurons suppress pain transmission in the spinal dorsal horn.
“The discovery of this new population of astrocytes reveals a new role of descending LC-NAergic neurons in facilitating spinal pain transmission,” explains Tsuda.
Considering these findings, suppressing signaling of these astrocytes by noradrenaline may enhance the effect of drugs for chronic pain.
To initially test this, the researchers genetically engineered mice in which response of astrocytes to noradrenaline was selectively inhibited and gave them duloxetine, an analgesic drug thought to increase levels of noradrenaline in the spinal cord by preventing uptake by descending LC-NAergic neurons.
Indeed, the modified mice exhibited an enhanced easing of chronic pain by duloxetine, further supporting the researchers’ proposed role of the astrocytes.
“Although we still need more studies with different drugs, this astrocyte population appears to be a very promising target for enhancing the therapeutic potential of drugs for chronic pain,” says Tsuda.
###
For more information about this research, see “Spinal astrocytes in superficial laminae gate brainstem descending control of mechanosensory hypersensitivity,” Yuta Kohro, Tsuyoshi Matsuda, Kohei Yoshihara, Keita Kohno, Keisuke Koga, Ryuichi Katsuragi, Takaaki Oka, Ryoichi Tashima, Sho Muneta, Takuya Yamane, Shota Okada, Kazuya Momokino, Aogu Furusho, Kenji Hamase, Takumi Oti, Hirotaka Sakamoto, Kenichiro Hayashida, Ryosuke Kobayashi, Takuro Horii, Izuho Hatada, Hidetoshi Tozaki-Saitoh, Katsuhiko Mikoshiba, Verdon Taylor, Kazuhide Inoue, and Makoto Tsuda, Nature Neuroscience (2020). https://doi.org/10.1038/s41593-020-00713-4
Research-related inquiries
Makoto Tsuda, Professor
Faculty of Pharmaceutical Sciences, Kyushu University
Contact information can also be found in the full release.
- TOP
- News
- Research Results
- New mechanism of pain control revealed































