研究成果 Research Results
- TOP
- News
- Research Results
- Inflammation and biology’s “ubiquitous” protein
Inflammation and biology’s “ubiquitous” protein
Ubiquitin-decorated ubiquitin-writing immune signaling regulator found to control inflammation 2020.12.10Research ResultsLife & Health
An international team of researchers have discovered a mechanism for how inflammation is regulated by a small modifier protein found so prolifically throughout regulatory processes in biology that it has earned the name ubiquitin.
In a testament to the ubiquity of ubiquitin, the newly discovered mechanism involves ubiquitin attaching to and modifying an immune signaling regulator known as HOIP, which itself functions as a ubiquitin ligase—a molecule that can “write” ubiquitin code onto other proteins through the process of ubiquitination.
Whereas past studies have pointed to HOIP being a critical regulator of inflammation, how HOIP itself is regulated to control inflammatory responses has been unclear.
The research group led by Fumiyo Ikeda of Kyushu University’s Medical Institute of Bioregulation and the Institute of Molecular Biotechnology of the Austrian Academy of Sciences (IMBA) in Vienna now reports that site-specific modification of HOIP with ubiquitin promotes inflammatory responses in cells and in mice.
Ikeda and her team had previously discovered that ubiquitin code written by HOIP and partner proteins HOIL-1L and SHARPIN regulates inflammation and cell death using mouse models, but the new study gives insight as to how HOIP itself is regulated in cells or in vivo.
In work published in The EMBO Journal, the researchers found that HOIP itself is modified by ubiquitin at multiple sites.
Using cells derived from genetically modified knockin mice in which HOIP is mutated, the researchers pinpointed one site in particular—lysine 784—as being important in regulating cellular response to Tumour Necrosis Factor (TNF), a critical mediator protein regulating immune responses that is part of a broader class of such proteins called cytokines.
When HOIP was mutated at that location, the cells could not properly mediate the series of processes controlled by TNF.
“It was a surprise when we observed that knockin mice with the HOIP mutant exhibited embryonic lethality if the partner protein SHARPIN is deficient,” says Ikeda. “We hope this research will ultimately contribute to understanding of the mechanisms of inflammation in humans.”
While more studies are still needed to clarify the processes in humans and determine what writes ubiquitin to HOIP, these findings provide new clues for understanding autoimmune disease mechanisms in which a ubiquitin code written on HOIP is involved.
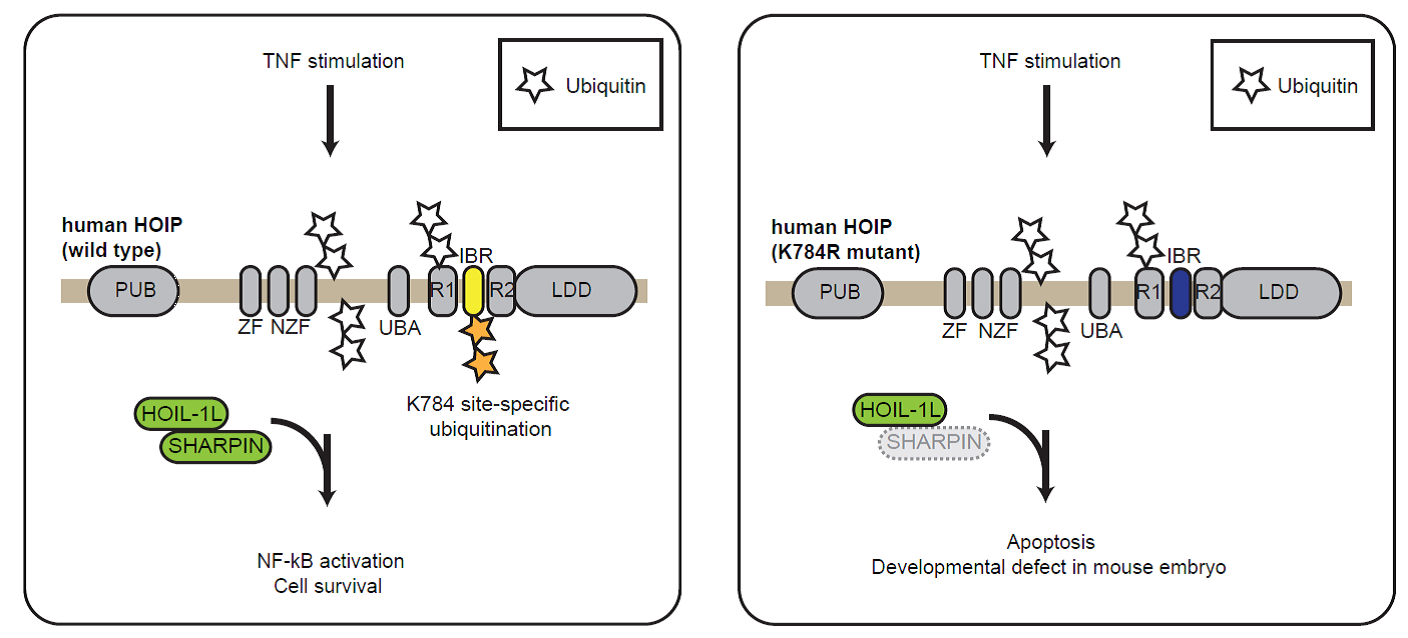
An E3 ubiquitin ligase HOIP forms a complex called LUBAC with SHARPIN and HOIL-1L. Researchers found that HOIP is ubiquitinated at multiple sites in cells, one of which at lysine 784 in the noncatalytic IBR domain plays a role in the regulation of apoptosis and NF-kB activation in cells (left). In mice, the equivalent ubiquitination site plays a collaborative role with SHARPIN to regulate TNF-dependent mouse development, and systemic inflammation (right). © 2020 Fennell et al.
###
For more information about this research, see “Site-specific ubiquitination of the E3 ligase HOIP regulates apoptosis and immune signaling,” Lilian M Fennell, Carlos Gomez Diaz, Luiza Deszcz, Anoop Kavirayani, David Hoffmann, Kota Yanagitani, Alexander Schleiffer, Karl Mechtler, Astrid Hagelkruys, Josef Penninger, and Fumiyo Ikeda, The EMBO Journal (2020). https://doi.org/10.15252/embj.2019103303
This release is also available in Japanese.
Research-related inquiries
Fumiyo Ikeda, Professor
Medical Institute of Bioregulation, Division of Inflammation and Proteostasis
Contact information can also be found in the full release.
- TOP
- News
- Research Results
- Inflammation and biology’s “ubiquitous” protein































