研究成果 Research Results
- TOP
- News
- Research Results
- Development of A New Catalytic Method for the Direct Synthesis of N-Unprotected α- and/or β- Tetrasubstituted Amino Acid Derivatives
Development of A New Catalytic Method for the Direct Synthesis of N-Unprotected α- and/or β- Tetrasubstituted Amino Acid Derivatives
2017.10.12Research ResultsPhysics & Chemistry
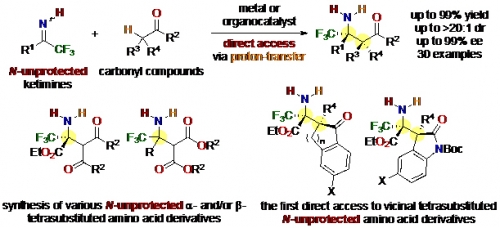
Unnatural amino acid derivatives with tetrasubstituted carbon stereocenters are useful for the synthesis of biologically active compounds because they have distinct properties compared with the natural ones. One of the most useful methods to synthesize these unnatural amino acid derivatives is catalytic asymmetric nucleophilic addition to N-protected ketimines, but unnecessary protection/deprotection steps are required to obtain N-unprotected amino acid derivatives.
To improve the synthetic efficiency, Professor Takashi Ohshima, Lecturer Hiroyuki Morimoto and co-workers succeeded in developing a new environmentally friendly method for the direct synthesis of N-unprotected amino acid derivatives. They found that Lewis acid catalysts were effective for the direct synthesis of various N-unprotected - and/or -tetrasubstituted amino acid derivatives, and a chiral organocatalyst enabled enantioselective variants including the first construction of contiguous tetrasubstituted carbon stereocenters for the synthesis of N-unprotected amino acid derivatives.
The above results have been published online on September 26, 2017 in Chemistry—A European Journal, an international general chemistry journal published by the Wiley, and selected as a Hot Paper of the Journal.
For more information about this research, see:
Direct access to N-unprotected - and/or -Tetrasubstituted Amino Acid Esters via Direct Catalytic Mannich-Type Reactions using N-unprotected Trifluoromethyl Ketimines
M. Sawa, K. Morisaki, Y. Kondo, H. Morimoto and T. Ohshima, Chem. Eur. J. Early View (DOI: 10.1002/chem.201703516).
Journal Reference
Direct Access to N-Unprotected α- and/or β-Tetrasubstituted Amino Acid Esters via Direct Catalytic Mannich-Type Reactions Using N-Unprotected Trifluoromethyl Ketimines, ,Chemistry - A European Journal, 10.1002/chem.201703516Research-related inquiries
- TOP
- News
- Research Results
- Development of A New Catalytic Method for the Direct Synthesis of N-Unprotected α- and/or β- Tetrasubstituted Amino Acid Derivatives































