研究成果 Research Results
- TOP
- News
- Research Results
- Organics glow longer and stronger without depending on rare metals
Organics glow longer and stronger without depending on rare metals
Versatile, organic glow-in-the-dark materials closer to matching the performance of their inorganic counterparts 2021.11.30Research ResultsPhysics & ChemistryMaterialsTechnology

Science is now one step closer to bringing the glow-in-the-dark effect often used in signs and watches to a wider variety of applications without the need for rare metals.
Applying a new strategy for combining carbon-based organic molecules, researchers at Kyushu University and the Okinawa Institute of Science and Technology (OIST) have dramatically improved the length and strength of the glow produced by the versatile materials, which have the potential to be more easily formed into paints and fibers than their rare-metal-containing counterparts.
The new work builds off the research group’s discovery in 2017 of the world’s first organic system for producing the glow-in-the-dark effect at room temperature by melting together two metal-free molecules.
Formally known as persistent luminescence, the glow-in-the-dark phenomenon is also often referred to as phosphorescence, though this term leads to ambiguity with another emission mechanism commonly found in organic materials.
While commercial glow-in-the-dark materials based on inorganic compounds containing rare-earth metals already achieve excellent performance, their inorganic nature often limits how they can be processed.
“Organic materials are more readily available than rare-metal-containing inorganic materials, and their solubility makes them easier to process,” explains Chihaya Adachi, professor and adviser on the research performed with Kazuya Jinnai at Kyushu University. “In addition to new applications for light-storing materials such as inks, films, and fibers, we expect organics to also enable bio-imaging applications in the future.”
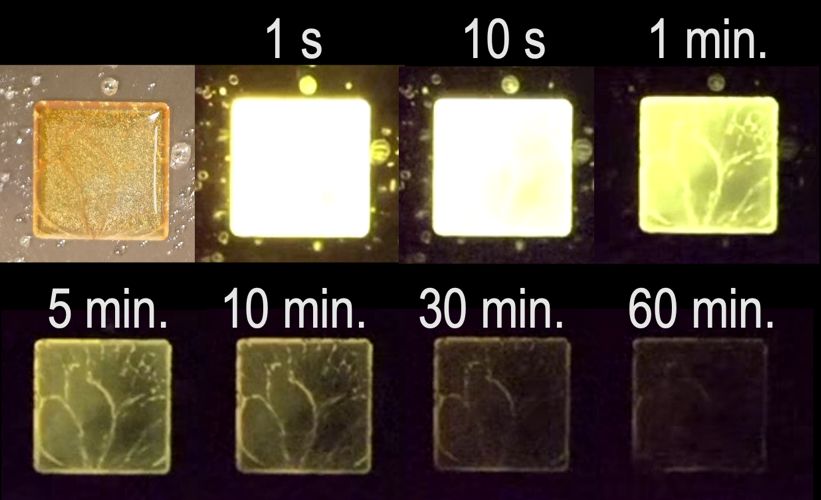
However, the length and strength of the emission from the organic alternatives was only about a one-hundredth of that of inorganic materials, and the glow quickly extinguished in the presence of oxygen.
“By changing our design strategy, we have now succeeded in improving the performance of organic persistent luminescence by about ten times over our previous report,” says Ryota Kabe, assistant professor and leader of the research at OIST with Zesen Lin.
At the heart of the emission mechanism is the absorption of light to excite a negatively charged electron into a state of higher energy.
Transfer of a lower-energy electron from a nearby donor molecule to fill the “hole” left behind by the excited electron leads to one molecule having one electron more than normal and the other one less—a situation known as a charge-transfer state.
Emission occurs when these excited electrons return to molecules missing an electron and give off their extra energy as light, so the key is to get the charges to separate by hopping between molecules and to slow down their eventual return to achieve a long-lived glow.
This time, the researchers chose a material combination in which the holes—the voids left by missing electrons—are what effectively hop between molecules. Holes are generally more stable and less reactive with oxygen, so the light emission was much longer in air than with their previous materials in which the excited electrons were mobile.
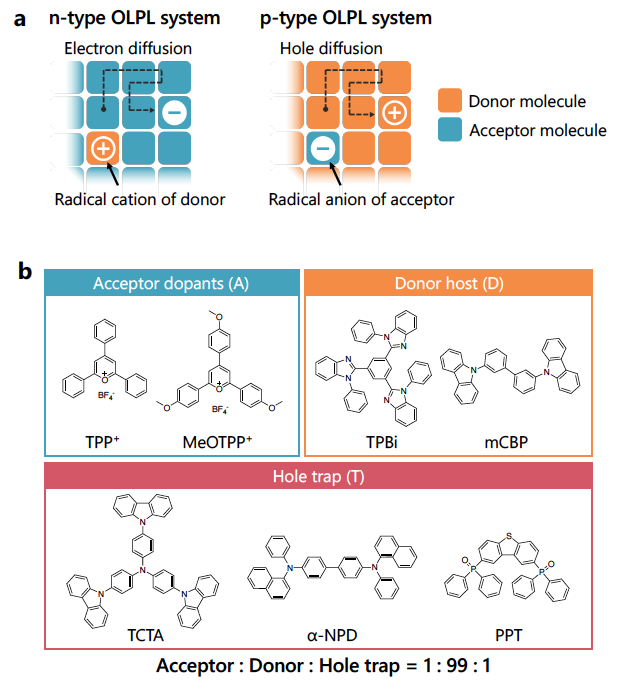
By employing an absorbing material that could be excited with lower-energy light, the materials were able to be energized with not only ultraviolet but also green and even orange light.
Additionally, the researchers were able to further stabilize the energy storage state by adding a third organic material that essentially traps the holes to further delay their return and extend the emission duration.
“We have now succeeded in achieving a longer duration and emission under atmospheric conditions,” comments Kabe. “While performance is still below that of inorganic materials, we hope to achieve performance that exceeds that of inorganics with further research.”
The researchers also hope that the organic materials developed in this research will help to expand and diversify sustainable industries without the need for rare metals.
“Time and time again, we are finding that precise control of organic charge-transfer materials enables the expression of a variety of emission properties, not only for glow-in-the-dark applications but also organic LEDs and lasers. I look forward to the new possibilities that a further deepening of the science will bring in the future,” concludes Adachi.
###
For more information about this research, see “Organic long-persistent luminescence stimulated by visible light in p-type systems based on organic photoredox catalyst dopants,” Kazuya Jinnai, Ryota Kabe, Zesen Lin, and Chihaya Adachi, Nature Materials (2021). https://doi.org/10.1038/s41563-021-01150-9
This release is also available in Japanese.
Acknowledgments
This research was supported by the Japan Science and Technology Agency (JST) ERATO Adachi Molecular Exciton Engineering Project (JPMJER1305), JST FOREST (JPMJFR201H), the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (JP18H02049, JP21H02020), JSPS Core-to-Core Program, the OIST POC Program, and the Kyushu University Platform of Inter-/Transdisciplinary Energy Research.
Research-related inquiries
Chihaya Adachi, Director
Center for Organic Photonics and Electronics Research (OPERA), Kyushu University
Contact information can also be found in the full release.
- TOP
- News
- Research Results
- Organics glow longer and stronger without depending on rare metals